Abstract
Astrocytes are critical for the development and function of synapses. There are notable species differences between human astrocytes and commonly used animal models. Yet, it is unclear whether astrocytic genes involved in synaptic function are stable or exhibit dynamic changes associated with disease states and age in humans, which is a barrier in understanding human astrocyte biology and its potential involvement in neurologic diseases. To better understand the properties of human astrocytes, we acutely purified astrocytes from the cerebral cortices of over 40 humans across various ages, sexes, and disease states. We performed RNA sequencing to generate transcriptomic profiles of these astrocytes and identified genes associated with these biological variables. We found that human astrocytes in tumor-surrounding regions downregulate genes involved in synaptic function and sensing of signals in the microenvironment, suggesting involvement of peritumor astrocytes in tumor-associated neural circuit dysfunction. In aging, we also found downregulation of synaptic regulators and upregulation of markers of cytokine signaling, while in maturation we identified changes in ionic transport with implications for calcium signaling. In addition, we identified subtle sexual dimorphism in human cortical astrocytes, which has implications for observed sex differences across many neurologic disorders. Overall, genes involved in synaptic function exhibit dynamic changes in the peritumor microenvironment and aging. These data provide powerful new insights into human astrocyte biology in several biologically relevant states that will aid in generating novel testable hypotheses about homeostatic and reactive astrocytes in humans.
SIGNIFICANCE STATEMENT Astrocytes are an abundant class of cells playing integral roles at synapses. Astrocyte dysfunction is implicated in a variety of human neurologic diseases. Yet our knowledge of astrocytes is largely based on mouse studies. Direct knowledge of human astrocyte biology remains limited. Here, we present transcriptomic profiles of human cortical astrocytes, and we identified molecular differences associated with age, sex, and disease state. We found that peritumor and aging astrocytes downregulate genes involved in astrocyte–synapse interactions. These data provide necessary insight into human astrocyte biology that will improve our understanding of human disease.
Keywords: aging, astrocyte, glioblastoma, human, maturation, sex
Introduction
Astrocytes are a major component of the CNS. Though astrocytes were long regarded as passive support cells, studies of murine astrocytes found they have active functions that are critical for the development and function of synapses. For example, astrocyte-secreted factors powerfully induce the formation of functional synapses in vivo and in vitro, which otherwise largely fails to occur (Banker, 1980; Ullian et al., 2001; Christopherson et al., 2005; Kucukdereli et al., 2011; Allen et al., 2012; Singh et al., 2016; Farhy-Tselnicker et al., 2017; Krencik et al., 2017; Stogsdill et al., 2017; Blanco-Suarez et al., 2018). In addition to important roles in synapse formation, astrocytes contribute to engulfment and elimination of synapses in development (Chung et al., 2013; Tasdemir-Yilmaz and Freeman, 2014; Vainchtein et al., 2018; Lee et al., 2021). Moreover, astrocytes maintain extracellular potassium levels (Kuffler et al., 1966; Olsen and Sontheimer, 2008; Kelley et al., 2018b) and participate in recycling neurotransmitters (Rothstein et al., 1996), thus maintaining homeostasis at synapses. There is now a variety of evidence showing that astrocytes help shape circuit functions and behavior (Nedergaard, 1994; Parpura et al., 1994; Halassa et al., 2009; Robel et al., 2015; Papouin et al., 2017; Dowling and Allen, 2018; Mu et al., 2019; Nagai et al., 2019; A. Y. Huang et al., 2020). Various groups have demonstrated that altering intracellular astrocyte signaling in vivo can induce abnormal behavior or correct phenotypic behavior in disease models (Chen et al., 2016; Ma et al., 2016; Ng et al., 2016; Kelley et al., 2018b; X. Yu et al., 2018, 2020; Ung et al., 2020; Nagai et al., 2021). Astrocytes are molecularly and functionally heterogeneous, potentially adapting to diverse roles they play in different brain regions (Tsai et al., 2012; Glasgow et al., 2014; Molofsky et al., 2014; Chai et al., 2017; John Lin et al., 2017; Morel et al., 2017; Miller et al., 2019; Diaz-Castro et al., 2021).
Astrocyte biology faces an added layer of complexity considering their significant dynamism in response to insult or injury (Poskanzer and Molofsky, 2018). Astrocytes undergoing reactive astrogliosis in response to a challenge can display stark morphologic changes, including hypertrophy and retraction of processes, in addition to a plethora of intracellular alterations (Sofroniew, 2020). Reactivity is observed in many neurologic disorders, including traumatic brain injury, stroke, epilepsy, and neurodegenerative diseases, and there appears to be disease-specific aspects to this response (Beach et al., 1989; Panickar and Norenberg, 2005; Binder and Steinhauser, 2006; Burda et al., 2016; Liddelow et al., 2017; X. Yu et al., 2020).
Given their many and varied roles in the CNS, astrocytes are frequently implicated in neurologic pathologies (Tian et al., 2005; Yamanaka et al., 2008; Ballas et al., 2009; Lioy et al., 2011; Molofsky et al., 2012; Krencik et al., 2015; Robel et al., 2015; Windrem et al., 2017; Laug et al., 2019). Recently, transcriptomic analysis of neuropsychiatric disease found astrocytic genes included in the gene signatures of autism spectrum disorder, bipolar disorder, and schizophrenia (Gandal et al., 2018a,b). Astrocyte reactivity is also prominent in several neurodegenerative diseases, including Alzheimer disease and Parkinson disease (Beach et al., 1989). In amyotrophic lateral sclerosis (ALS), reactive astrogliosis occurs around degenerating motor neurons, and this reactivity precedes motor neuron death in the rat SOD1 model of ALS (Howland et al., 2002). Further investigation found that overexpressing GLT1 in astrocytes improved neuronal survival and delayed disease onset in the mSOD1 mouse model of ALS (Guo et al., 2003).
Our understanding of human astrocytes significantly trails our knowledge of murine astrocytes (de Majo et al., 2020). Although the majority of major astrocyte functions appear to be shared between mice and humans, it is still imperative to narrow this gap in knowledge as researchers continue to identify important differences between these species in the CNS. First, human astrocytes are notably larger with more elaborate branching than rodent astrocytes in vivo and in vitro (Oberheim et al., 2006, 2009; Zhang et al., 2016). At the molecular level, previous characterization of human and mouse astrocyte transcriptomes found many genes specifically expressed by human astrocytes (Zhang et al., 2016; Li et al., 2021). At a functional level, behavioral differences were observed in vivo when human glial progenitors were transplanted into mice (Han et al., 2013). Animal studies have produced, and continue to produce, a remarkable body of knowledge concerning the many vital astrocytic functions in the brain (e.g., synapse formation, circuit functions). It is because animal models clearly demonstrate the importance of astrocyte biology that complementary analysis is also required in humans.
Given the importance of astrocytes in synaptic function, a key question that needs to be answered is whether genes involved in astrocyte–synapse interactions are stable or exhibit dynamic changes associated with disease states and age in humans. With the advent of improved astrocyte purification methodologies, we can now extract highly pure populations of human astrocytes by targeting the astrocytic cell surface protein HepaCAM using antibodies. By using an immunopanning technique, we previously published human astrocyte transcriptomes of 12 human cortical samples between the ages of 8 and 63 years old (Zhang et al., 2016). In this study, we acutely purified samples from over 40 patients, which now include astrocytes from healthy and diseased brain regions. For the first time, we are also presenting samples under the age of 8, allowing for analysis of human astrocyte maturation, as well as other biological variables of interest. Here, we describe some of the first transcriptomic data of human astrocytes in the tumor microenvironment, as well as changes in astrocyte gene expression associated with maturation, aging, and sex. Among our findings, we see downregulation of synaptic genes in peritumor astrocytes as well as aging astrocytes.
Materials and Methods
Human tissue
Human tissue was obtained with informed consent and the approval of the UCLA Institutional Review Board. We obtained tissue primarily from brain surgeries at UCLA to treat epilepsy and tumors, plus one postmortem sample with short postmortem interval (<18 h). All samples were from the cerebral cortex, primarily from the temporal lobe (n = 31), but several samples came from the frontal (n = 9) or parietal lobes (n = 5), or the insula (n = 2). Tissue was immersed in 4°C media (saline or Hibernate-A medium) before transfer to the laboratory for dissection and dissociation. Six samples were obtained from surgeries offsite, which were shipped overnight in 4°C media for dissection and dissociation in the laboratory. The final cohort includes 7 peritumor samples, 30 epilepsy samples, and 12 controls totaling 49 samples from 41 patients (see Extended Data Fig. 1-1). No affected samples came from the same patient that provided a control sample.
Vertebrate animals
All mouse experimental procedures were performed with approval from the UCLA Chancellor's Animal Research Committee in compliance with all federal and state laws and policies. For ISH of mouse brain tissue, we used mice at postnatal day 71 (2 females, 1 male) from a C57/BL6 FVB mixed background.
Purification of human astrocytes
Human astrocytes were purified using immunopanning, as described by Zhang et al. (2016). Briefly, we dissected gray matter from cortical tissue and enzymatically digested the tissue with papain (20 units/ml) for 80 min at 34.5°C. We then rinsed the tissue in a protease inhibitor solution. We gently triturated the tissue to generate a single-cell suspension, and we passed the cells over a series of plastic Petri dishes that were precoated with antibody. The cell suspension was incubated at room temperature for 10–15 min on each dish, which consisted of two plates with anti-CD45 antibody (BD Pharmingen 198 550539) to deplete microglia, two plates with GalC hybridoma to deplete oligodendrocytes and myelin, and one plate with anti-Thy1 (BD Pharmingen 200 550402) to deplete neurons. Finally, the astrocyte-enriched cell suspension was incubated for 20 min at room temperature on a dish coated with anti-HepaCAM antibody (R&D Systems, MAB4108) to bind astrocytes. We washed the bound astrocytes with PBS to remove contaminants, and we immediately harvested RNA by applying 700 µl of TRIzol solution (Thermo Fisher Scientific, 15596018). TRIzol solution was then flash frozen in liquid nitrogen and stored at −80°C to await RNA purification. The total time from receiving tissue to storing astrocyte RNA took ∼4 h.
RNA library construction and sequencing
RNA was extracted from frozen TRIzol using the miRNeasy kit (QIAGEN, 217004), according to the manufacturer's protocol. We checked RNA quality with the 2200 TapeStation System (Agilent Technologies, G2964AA) and the RNA high sensitivity assay (Agilent Technologies, 5067-5579). All RNA integrity numbers were ≥ 6.5, except RNA samples that were not concentrated enough for accurate measurement. We then used the Nugen Ovation RNAseq System V2 (Nugen 7102-32) to generate cDNA libraries, and we fragmented the cDNA using a Covaris S220 focused-ultrasonicator (Covaris, 500217). We amplified and prepared these libraries for sequencing with the NEB Next Ultra RNA Library Prep Kit (New England Biolabs, E7530S) and NEBNext multiplex oligos for Illumina (NEB, E7335S). We performed end repair and adapter ligation, and we amplified the final libraries using 10 cycles of PCR. The sequencing libraries were verified using the TapeStation D1000 assay (Agilent Technologies, 5067-5582). Indexed libraries were pooled and sequenced using the Illumina HighSeq 4000 Sequencer and obtained 18.9 ± 1.6 million (mean ± SEM) single end, 50 bp reads across four batches.
Read alignment and quantification
We mapped reads using the STAR package (Dobin et al., 2013) and genome assembly GRCh38 (Ensembl, release 91), and obtained 77.0 ± 5.8% (mean ± SD) uniquely aligned reads in all samples. Reads were counted using the HTSeq package (Anders et al., 2015), and reads were subsequently quantified by reads per kilobase of transcript per million mapped reads (RPKM) using EdgeR-limma packages in R (Extended Data Fig. 1-2).
Differential gene expression analysis of disease and sex
We analyzed differential gene expression of disease and sex using the DESeq2 package in R (see Extended Data Figs. 2-1, 4-1, and 6-1) (Love et al., 2014). In this analysis, we included all samples and used the following command to create our linear model: ∼ factor(Diagnosis) + Age + factor(Sex) + MicroPC + Batch, where Diagnosis was a factor with values [Control, Peritumor, Epilepsy], Age was a numeric value in years, Sex was a factor with values [Male, Female], and MicroPC was numeric value measuring microglia contamination. To calculate the “microPC,” we first determined the gene expression of microglia-specific genes (>10× enriched in microglia vs astrocytes) in all samples, using the data from Zhang et al. (2016). Then, we performed principal component analysis (PCA) using the prcomp function in R on the scaled microglia gene expression, and we took the first principal component as a summary measure of microglial gene expression in each sample. Results were cross-checked with leave-one-out validation, where the analysis was reiterated with the removal of one sample in each round for a total of 49 iterations. To determine how robust the analysis is to the effect of brain region, we reran the analysis using only temporal lobe tissue (see “Results” in Extended Data Fig. 2-2). We further assessed the effect of brain region by performing differential gene expression analysis of region in samples from temporal lobe and frontal lobe (peritumor samples excluded; see Extended Data Fig. 2-3). We used the linear model: ∼ factor(Region) + Age + factor(Sex) + MicroPC + Batch.
Analysis of human aging genes
To identify genes associated with aging astrocytes, we began with genes significantly associated with Age in the DESeq2 analysis of disease and sex, as described above. In order to separate genes that change in aging (i.e., later life) from genes that change in development (early life), we categorized samples in three categories, excluding peritumor samples: 0–21 years old (n = 34), 21–50 years old (n = 3), and 50+ years old (n = 5). We compared younger adults (21–50) to older adults (50+), and we narrowed the gene list to those with an average expression > 0.01 RPKM and 1.5-fold differences in the average expression between groups. This yielded a list of 394 gene entries, 277 of which were protein-coding (see Extended Data Fig. 5-1).
Analysis of human astrocyte maturation
We analyzed differential gene expression across astrocyte maturation using a two-step process. First, we performed DESeq2 on samples ≤ 21 years old, excluding peritumor samples (n = 35). Model: ∼ factor(Diagnosis) + Age + factor(Sex) + MicroPC + OligPC + factor(Batch). The “oligPC” was calculated in the same manner as the microPC using gene expression of oligodendrocyte-specific genes identified using data from Zhang et al. (2016). While it is necessary to use cell type-specific filters to exclude contamination, excluding oligodendrocyte-enriched genes may obscure potential lineage relationships between astrocytes and oligodendrocytes (Weng et al., 2019). Thus, we only used these filters for differential expression analysis and included the unfiltered complete dataset in Extended Data Figure 1-2. Finally, we filtered out genes that were 10× enriched in human neurons over human astrocytes, using data from Zhang et al. (2016). This yielded 1463 gene entries significantly associated with maturation. We also performed a leave-one-out reanalysis to assess the robustness of the maturation gene expression findings (see Extended Data Fig. 2-2).
The DESeq2 analysis included samples as young as 7 months old, but we could capture changes from earlier stages in development by using our recently published transcriptomic profiles of fetal human astrocytes (Li et al., 2021). We compared 4 fetal samples with our near-adult human samples between 13 and 21 years old (excluding peritumor, n = 11). For each sample, gene expression was converted to a percentile, where the most highly expressed gene = 1 and the least expressed gene = 0. Next, we conducted parallel t tests with a Benjamini–Hochberg correction for multiple tests, performed in GraphPad Prism version 8. This generated over 10,000 hits. Finally, we combined the two gene lists using an equal number of genes from each list (i.e., we filtered results from the second analysis to the 1463 top hits by p-value to match the first analysis), producing a final list of 2749 genes associated with human astrocyte maturation (see Extended Data Fig. 4-2).
Analysis of disease-associated genes
We tested whether peritumor or maturation gene signatures were enriched with disease-associated genes. We obtained gene lists for various neurologic diseases from a curated database, Phenopedia (W. Yu et al., 2010). Using these gene lists, we performed Fisher's exact tests on differentially expressed genes in peritumor astrocytes and corrected for multiple comparisons using the Benjamini–Hochberg method. The results are in Extended Data Figure 2-4.
Mouse aging genes and human comparison
We accessed mouse astrocyte RNAseq data from the BioProject database (www.ncbi.nlm.nih.gov/bioproject; accession no. PRJNA417856) (Clarke et al., 2018). We downloaded FASTQ files of sequenced cortical astrocytes at ages postnatal day 7 (n = 3), 10 weeks (n = 3), and 2 years (n = 2). Reads were aligned with STAR 2.6.0 and genome assembly GRCm38 (Ensembl release 100). All samples had >70% uniquely mapped reads. We used HTSeq to generate counts, and then we determined differential gene expression between two ages (day 7 vs 10 weeks & 10 weeks vs 2 years) using DESeq2. Model = ∼ factor(Age), where Age is a binary factor.
We used the STRING database (www.string-db.org) to find and visualize protein–protein interactions in human and mouse aging-associated genes. For mouse aging genes, we combined the results of three studies of mouse astrocytes (Boisvert et al., 2018; Clarke et al., 2018; Ximerakis et al., 2019).
Gene ontology (GO)
To identify patterns in our various differentially expressed gene lists, we used the online platform Metascape (www.metascape.org) (Zhou et al., 2019). We input all protein-coding genes from our gene sets, and conducted an enrichment analysis with default settings, with the following adjustments: reference datasets were limited to GO datasets (Molecular Functions, Biological Processes, and Cellular Components), and we defined a list of background genes (i.e., the set of genes expressed in astrocytes that are included in this analysis) as follows: for human analyses, the background gene list consisted of all genes with expression ≥0.05 RPKM in ≥30% of our samples. For mouse analyses, the background list consisted of genes with ≥0.05 RPKM in ≥30% in the mouse samples from Clarke et al. (2018).
We used Fisher's exact test to individually test differentially expressed genes in peritumor astrocytes for enrichment of GO gene sets that were reportedly found in tumor-core astrocytes (Heiland et al., 2019): positive regulation of receptor signaling via JAK/STAT (GO:0046427/GO:2000366), negative regulation of receptor signaling via JAK/STAT (GO:0046426, GO:2000365), interleukin-6-mediated signaling pathway (GO:0070102), and response to interferon γ (GO:0034341).
Pro- and anti-inflammatory genes were identified using GO annotations for positive regulation of inflammatory response (GO:0050729) and negative regulation of inflammatory response (GO:0050728) (Ashburner et al., 2000; Carbon et al., 2009; Gene Ontology Consortium, 2021).
RNAscope ISH
RNAScope ISH was performed on fresh frozen human tissue collected from surgeries and fresh frozen mouse tissue harvested after a 10 min transcardial perfusion of PBS. Tissues were embedded in OCT compound (Fisher Scientific 23-730-571) and cut into 20- to 30-μm-thick sections. RNAScope Multiplex Fluorescent V2 Assay (ACDBio 323100) was performed per manufacturer's protocols. Probes were purchased from ACDBio for human and mouse SLC1A3 and TLR4. Images were captured using the Carl Zeiss LSM 800 confocal microscope at equal power and exposure across all samples stained with the same set of probes. Photoshop version 22.1 was used to enhance brightness.
Statistical analyses
Differential gene expression was analyzed using DESeq2. Enrichment of GO terms was analyzed using Metascape. Enrichment of disease-associated genes and peritumor genes was analyzed with Fisher's exact test in R using fisher.text(). All analyses are detailed under the corresponding sections above.
Data deposition
All human RNAseq data are deposited on the Gene Expression Omnibus at GSE168375.
Results
Purification of human cortical astrocytes in health and disease
We obtained human cortical tissue from patients undergoing neurologic surgery. Our final analysis includes 49 samples from 41 patients, with ages ranging from 7 months to 65 years old. Twelve of these samples were taken from normal regions of cortex that were resected en route to deep-seated pathologies. Nine of these normal specimens were obtained during surgery for deep epileptogenic foci. In these cases, we confirmed that normal specimens taken did not include abnormal-appearing tissue using MRI-registered frameless stereotaxy, did not show abnormal interictal activity on invasive electrode recordings, and did not demonstrate abnormal histopathologic findings. Two other cases from which normal specimens were collected were encapsulated, benign tumors, where normal-appearing brain tissue based on MRI-registered frameless stereotaxy was collected outside a 1 cm margin from the tumor. Finally, one normal tissue specimen was obtained from a patient at the time of death from a cardiac condition, without other intracranial pathologies. A similar cohort of control human astrocytes were sequenced and analyzed previously, where the authors characterized the baseline characteristics of the human astrocyte transcriptome (Zhang et al., 2016); of note, we used remaining RNA from 7 of these samples in this study. From this point forward, we refer to normal brain tissue samples from these sources as “controls.”
In the current study, we sought to compare normal astrocytes against disease-affected astrocytes. Affected samples included in the analysis fall into two categories of diagnosis: (1) 30 included brain tissue showing epileptiform activity on intraoperative electrode recordings surrounding resected focal cortical dysplasia (FCD), a developmental form of epilepsy; and (2) 7 included brain tissue immediately surrounding a brain tumor (including glioblastoma, low-grade glioma, and metastatic breast cancer) based on MRI and visual inspection at the time of surgery (referred to here as “peritumor”). Specific pathologic diagnoses are presented in Extended Data Figure 1-1.
We purified human cortical astrocytes using immunopanning (Fig. 1A). We removed white matter and generated a single-cell suspension with mechanical and enzymatic digestion. The single-cell suspension passes over antibody-coated Petri dishes that bind contaminating cell types with cell type-specific antigens. This immunopanning protocol uses anti-CD45 antibodies to pull down myeloid cells (i.e., microglia and macrophages), anti-GalC hybridoma cell supernatant to pull down oligodendrocytes and myelin debris, and anti-Thy1 antibodies to bind neurons. Finally, the enriched suspension passes onto a dish coated in anti-HepaCAM antibodies, a cell-surface glycoprotein enriched in astrocytes. We harvested the astrocyte RNA from this dish and used it to perform RNA sequencing (RNAseq). The sequenced samples show high expression of astrocyte marker genes, such as GFAP and SLC1A2, and extremely low expression of neuronal, myeloid, and endothelial genes. There are only slight traces of some oligodendrocyte-lineage marker genes (Fig. 1B). Using immunopanning, we obtained RNA highly enriched for human cortical astrocytes in healthy and diseased states for bulk RNAseq (Extended Data Fig. 1-2).
Figure 1.
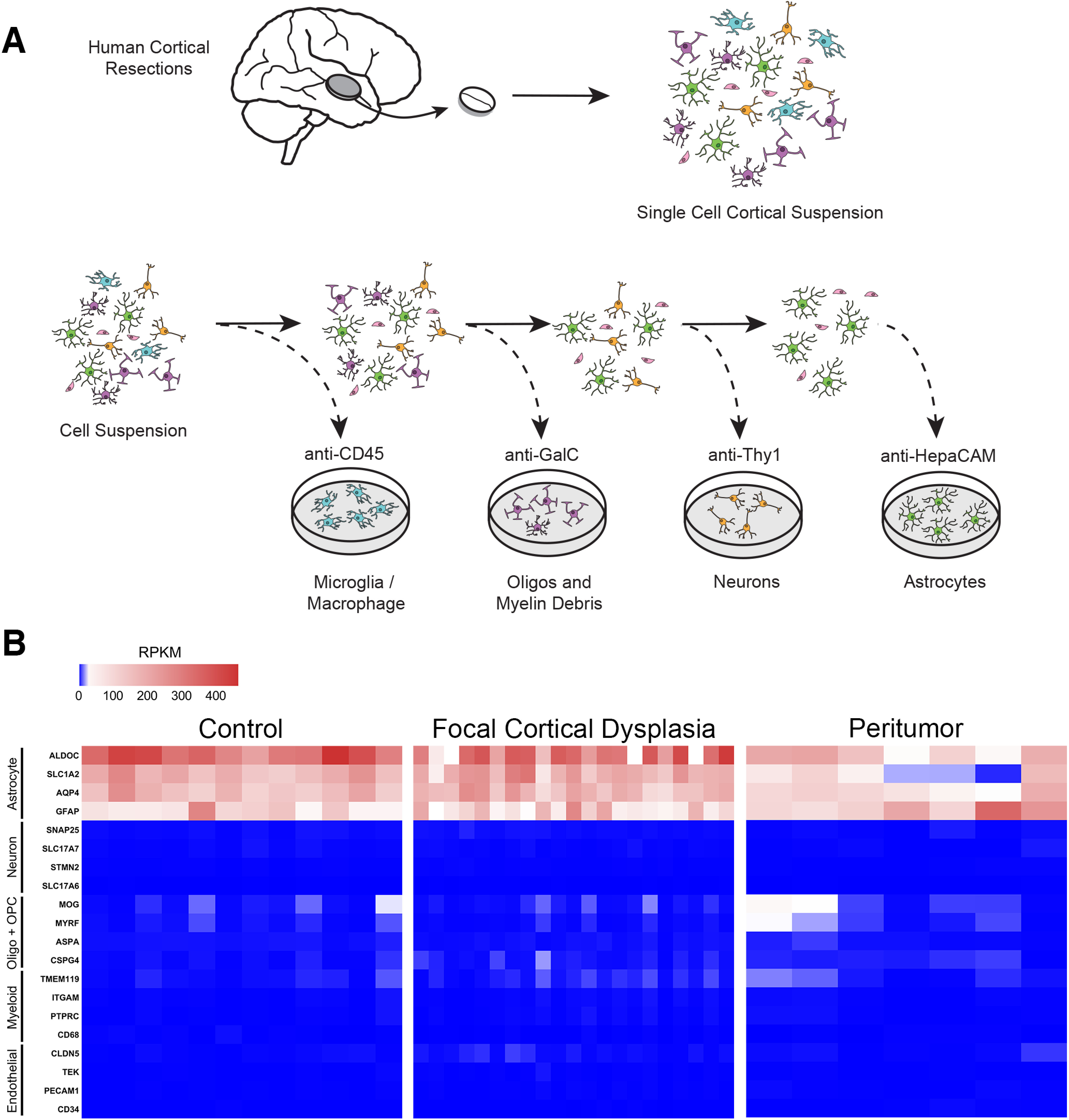
Acute purification of human astrocytes from cerebral cortex. A, Diagram of human astrocyte purification by immunopanning. Surgically resected tissue underwent enzymatic digestion and gentle mechanical digestion to generate a single-cell suspension. These cells were passed over a series of plates coated with cell type-specific antibodies to deplete microglia, oligodendrocyte-lineage cells, and neurons before finally passing to a plate that specifically binds astrocytes using an anti-HepaCAM antibody. B, Heatmaps represent the expression of cell type-specific genes in RPKM after RNA sequencing of immunopanned astrocytes. All samples are highly enriched in astrocytic genes (red), with little to no expression of gene markers for neurons, myeloid cells (i.e., microglia or macrophages), oligodendrocyte-lineage cells, or endothelial cells. Detailed sample info and total gene expression are detailed in Extended Data Figures 1-1 and 1-2.
Human astrocyte metadata. Download Figure 1-1, XLSX file (12.8KB, xlsx) .
Human Astrocyte Gene Expression (RPKM). Download Figure 1-2, XLSX file (26.7MB, xlsx) .
Glioblastoma cells infiltrate surrounding brain tissue. To determine whether our purified astrocytes from the peritumor regions are bona fide astrocytes or infiltrating glioblastoma cells, we examined the expression of a glioblastoma marker gene AVIL (Xie et al., 2020) and did not detect expression in our peritumor astrocyte samples (Extended Data Fig. 1-2). Furthermore, we compared gene expression of astrocytes surrounding glioblastoma tissue (infiltrating) and astrocytes surrounding low-grade glioma or metastatic tumors (noninfiltrating). Peritumor astrocyte signature genes described below are not more highly expressed by glioblastoma-surrounding astrocytes than noninfiltrating tumor-surrounding astrocytes (Extended Data Fig. 1-2). Although we cannot rule out contamination from a small number of infiltrating glioblastoma cells, the gene signatures of peritumor astrocytes are likely from predominantly bona fide astrocytes instead of infiltrating cells.
Peritumor astrocytes downregulate genes involved in synaptic function and genes encoding cell surface receptors
After sequencing RNA from human astrocytes in FCD and peritumor regions, we used differential gene expression analysis to examine their molecular signatures using the analysis package DESeq2 in R. We used a linear model that included variables for diagnosis, sequencing batch, age, and sex. To control for potential variance from low-level microglial contamination, we included an additional variable that quantified microglial marker gene expression by performing PCA on microglial marker gene expression in our dataset. Including the first principal component in the linear model effectively eliminated significant differential expression of microglial genes.
First, we examined the effect of the brain tumor microenvironment on astrocyte gene expression. Brain tumors drive considerable changes in the surrounding microenvironment, and astrocytes themselves are known to readily change state in response to a variety of stimuli (Raore et al., 2011; Quail and Joyce, 2017). However, transcriptomic changes of peritumor astrocytes in humans have not been reported, to the best of our knowledge. Using our DESeq2 pipeline, we found 3131 genes differentially expressed in peritumor astrocytes, providing a new resource for elucidating astrocytic changes in the brain tumor microenvironment (Extended Data Fig. 2-1). The vast majority of these findings were robust under leave-one-out validation (Extended Data Fig. 2-2) where the analysis was reiterated with the removal of one sample in each round.
Synaptic gene signatures in peritumor astrocytes
Many genes related to synaptic function are downregulated in the peritumor region (Fig. 2A–D), such as the glutamate transporters SLC1A2 and SLC1A3, which take up the excitatory neurotransmitter glutamate from the synaptic cleft and maintains excitation-inhibition balance in the brain (Yang et al., 2009). SLC1A2-KO mice suffer from epileptic seizures and die prematurely (Tanaka et al., 1997). Also downregulated is the gene KCNJ10 encoding the inwardly rectifying potassium channel Kir4.1, which takes up potassium from the extracellular space after neuronal firing, buffers potassium levels in the astrocytic syncytium, and modulates neuronal excitability (Kofuji and Newman, 2004). Patients with mutations in the KCNJ10 gene suffer from seizure disorders (Reichold et al., 2010). A large proportion of human patients with brain tumors also suffer from epileptic seizures, which reduce their quality of life and sometimes cause death (Englot et al., 2016). Our observation of strong reductions of SLC1A2, SLC1A3, and KCNJ10 in peritumor astrocytes suggest potential involvement of astrocytes in tumor-associated seizures and reveal astrocytes as novel potential targets for treating these seizures. A study in a rat model of glioma supports the feasibility of this approach (Sattler et al., 2013). Furthermore, astrocyte-secreted molecules that regulate synapse formation and maturation (SPARCL1, CHRDL1, and GPC5) are also downregulated in peritumor astrocytes (Fig. 2B,D). Together, these findings suggest decreased support of synapses in the tumor microenvironment that could contribute to dysregulation of synaptic activity and emergent clinical symptoms, such as seizures. Upregulated genes in peritumor astrocytes include the reactivity-associated genes GFAP, TIMP1, and VIM, suggesting that astrocytes in the peritumor microenvironment are reactive in humans.
Figure 2.

Transcriptomic signature of human astrocytes in the peritumor microenvironment. A, Volcano plot represents differential gene expression in human peritumor astrocytes versus controls. Red represents p < 0.05. Full differential gene expression in Extended Data Figure 2-1; cross-validation in Extended Data Figure 2-2; effects of brain region in Extended Data Figure 2-3. B, Bar plots of astrocyte genes with changing expression in the peritumor microenvironment. Error bars indicate standard deviation. C, Selected GO terms that are significantly enriched in upregulated (left) and downregulated (right) genes in peritumor astrocytes. Dashed lines indicate p = 0.05. Full GO results in Extended Data Figure 2-6. Analysis of synaptic genes and disease-associated genes in Extended Data Figure 2-4. D, Heatmaps of differential gene expression in peritumor astrocytes related to (top) synaptic function, (middle) astrocyte reactivity, and (bottom) mature astrocyte markers. E, Heatmaps of extracellular matrix genes with increased (red) or decreased (blue) expression in peritumor astrocytes, all significant at p < 0.05. F, Normalized gene expression of anti-inflammatory (top) and pro-inflammatory (bottom) genes that are differentially expressed in peritumor astrocytes (p < 0.05). G, Normalized gene expression of plasma membrane receptors that are differentially expressed in peritumor astrocytes (p < 0.05). Full gene list in Extended Data Figure 2-5. H, Normalized gene expression of the top 50 astrocyte marker genes, identified by Kelley et al. (2018a), in peritumor and control astrocytes. *p < 0.05. **p < 0.01. ***p < 0.001.
Differential Gene Expression in Peritumor Astrocytes. Download Figure 2-1, XLSX file (186.1KB, xlsx) .
Cross-Validation of Differential Gene Expression; Peritumor digital gene expression leave-one-out. Download Figure 2-2, XLSX file (367.3KB, xlsx) .
Differential Gene Expression by Brain Region (Temporal versus Frontal Cortex). Download Figure 2-3, XLSX file (14.5KB, xlsx) .
Associations of Disease-Linked Genes and Peritumor Astrocyte Genes. Download Figure 2-4, XLSX file (11.8KB, xlsx) .
Astrocyte Receptor Genes. Download Figure 2-5, XLSX file (14.9KB, xlsx) .
GO Pathways Upregulated in Peritumor Astrocytes. Download Figure 2-6, XLSX file (62.8KB, xlsx) .
Cell surface receptors in peritumor astrocytes
Cell surface receptors mediate sensing of signals in the microenvironment. To examine the expression of genes encoding receptors located on the plasma membrane by peritumor astrocytes, we used a list of experimentally validated and in silico predicted genes encoding cell surface receptors (Bausch-Fluck et al., 2018). We determined the complete set of transmembrane receptors present in human astrocytes (Extended Data Fig. 2-5). We found 157 transmembrane receptor genes expressed by human astrocytes (average RPKM >1). Of these genes, peritumor astrocytes downregulate one-fourth (40 of 157) and upregulate only 3 of 157, suggesting that peritumor astrocytes are impaired in their ability to receive and respond to external cues (Fig. 2G).
Synaptic genes and cell surface receptors downregulated in peritumor astrocytes are associated with psychiatric disease risk
Having discovered genes upregulated and downregulated in peritumor astrocytes, we next assessed whether these genes are involved in other diseases. We used the Phenopedia dataset for genes associated with various neurologic diseases (W. Yu et al., 2010) and tested whether genes upregulated and downregulated in peritumor astrocytes are significantly enriched in genes associated with the risk of each neurologic disorder using Fisher's exact test followed by correction for multiple comparisons (see Materials and Methods). Genes that were downregulated in peritumor astrocytes were enriched for genes with genetic links to several neurologic diseases (Extended Data Fig. 2-4). Interestingly, these associations existed primarily among psychiatric disorders (bipolar disorder, schizophrenia, mood disorders, obsessive-compulsive disorder, depressive disorder, and anxiety disorder). In addition, genes downregulated in peritumor astrocytes also significantly overlap with Alzheimer's disease risk genes, but not with other neurodegenerative disorders (Parkinson disease, ALS, frontotemporal dementia, and Huntington disease).
Next, we asked whether synaptic and receptor genes contributed to the association between peritumor genes and genes associated with psychiatric disease. We tested whether synaptic genes and receptor genes downregulated in peritumor astrocytes were enriched in genes associated with risks of the aforementioned six psychiatric disorders and Alzheimer's disease. We found that both synaptic and receptor genes downregulated in peritumor astrocytes are enriched in genes associated with all six psychiatric disorders, whereas Alzheimer's disease only associated with synaptic genes (Extended Data Fig. 2-4). We conclude that peritumor astrocytes downregulate receptor and synaptic genes that are associated with psychiatric disease risk, highlighting the potential importance of this core group of astrocytic genes in neural circuit development and function.
GO of peritumor astrocyte signatures
To find larger patterns in the data, we performed pathway analysis with the online tool Metascape to identify GO terms that are enriched in our gene lists (Extended Data Fig. 2-6). Among upregulated genes, we found highly significant enrichment of GO terms related to cell cycle and protein translation, consistent with the presence of proliferative reactive astrocytes in the peritumor region. Meanwhile, downregulated genes were enriched for an array of functional terms related to synaptic function as well as cation transport (Fig. 2C), further supporting the hypothesis that peritumor astrocytes are defective in supporting or participating in normal synaptic signaling. Interestingly, both upregulated and downregulated gene lists are enriched for extracellular matrix genes (Fig. 2E), which is notable considering the importance of extracellular remodeling in tumor expansion and migration as well as synaptic plasticity (Nguyen et al., 2020; Winkler et al., 2020). Broadly, we see peritumor astrocytes alter extracellular matrix gene expression while upregulating genes necessary for cell division and translation, and downregulating expression of genes related to synaptic transmission and ionic homeostasis, revealing potential contribution of astrocytes to neural circuit dysfunction associated with brain tumors.
Peritumor astrocytes differ from tumor-core astrocytes
To assess whether peritumor astrocytes resemble tumor-core-associated astrocytes, we compared our peritumor astrocyte dataset to a previously published tumor-core-associated astrocyte dataset (Heiland et al., 2019). Heiland et al. (2019) reported that tumor-core astrocytes contribute to an immunosuppressive environment in part because of increased JAK/STAT pathway activation. Among peritumor astrocytes, however, we did not find significant enrichment of the JAK/STAT pathway among differentially expressed genes. We also failed to find enrichment of interferon γ response genes and interleukin-6 response genes, which were also identified by Heiland et al. (2019). When we examined pro- and anti-inflammatory genes that are differentially expressed in peritumor astrocytes, we found that the majority of pro-inflammatory genes were upregulated (10 of 11; Fig. 2F), and the majority of anti-inflammatory genes were downregulated (16 of 26; Fig. 2F). Together, these findings suggest a contrast between an anti-inflammatory signature of tumor-core astrocytes, and an at least partly pro-inflammatory signature of peritumor astrocytes.
Peritumor astrocytes attenuate core astrocytic genes
To assess whether peritumor astrocytes may lose normal astrocyte function, we examined genes highly expressed by astrocytes and found that peritumor astrocytes downregulate several known markers of mature astrocytes (Fig. 2D). Furthermore, we examined a list of the top 50 astrocyte markers identified in a meta-analysis of human gene expression (Kelley et al., 2018a). Peritumor astrocytes significantly downregulated 41 of 50 genes, with 50 of 50 trending downward (Fig. 2H). These results are consistent with a possible loss of normal astrocytic functions in the peritumor microenvironment.
Toll-like receptor 4 (TLR4) is expressed by human astrocytes and not mouse astrocytes
To assess changes of peritumor astrocytes using an orthogonal approach, we performed ISH with RNAscope. Based on RNAseq, we found that TLR4 was downregulated in peritumor astrocytes. TLR4 is a member of the TLR family of pattern recognition receptors, which recognize pathogen-associated molecular patterns and initiate innate immune responses (Park and Lee, 2013). Specifically, TLR4 encodes a transmembrane protein that binds bacterial lipopolysaccharides and triggers innate immune responses to bacterial infection. Moreover, TLR4 also recognizes endogenous ligands, such as heat shock proteins and lipoproteins from damaged cells (Vaure and Liu, 2014). Previous studies in mice found that TLR4 was highly enriched in myeloid cells, such as microglia in the brain, but we detected significant mRNA expression of TLR4 in astrocytes in humans (Zhang et al., 2016; current study). To directly compare TLR4 expression between species, we performed RNAscope ISH on human and mouse cortical tissue. Whereas a small minority of mouse astrocytes expressed TLR4 (7%, 4 of 58 cells, n = 3), a majority of human astrocytes showed expression of TLR4 (62%, 18 of 29 cells, n = 2; Fig. 3A,B). Even more strikingly, TLR4+ astrocytes in humans contain larger numbers of TLR4+ mRNA puncta than did TLR4+ astrocytes in mice (Fig. 3C). Although the small sample size does not provide a definitive conclusion, we find evidence of human-specific expression of TLR4 in astrocytes that corroborates findings from RNAseq, suggesting enhanced ability of human astrocytes to detect TLR4 ligands, such as signals from bacteria and damaged cells, compared with mouse astrocytes. Furthermore, this mode of signaling is notably reduced in peritumor astrocytes.
Figure 3.
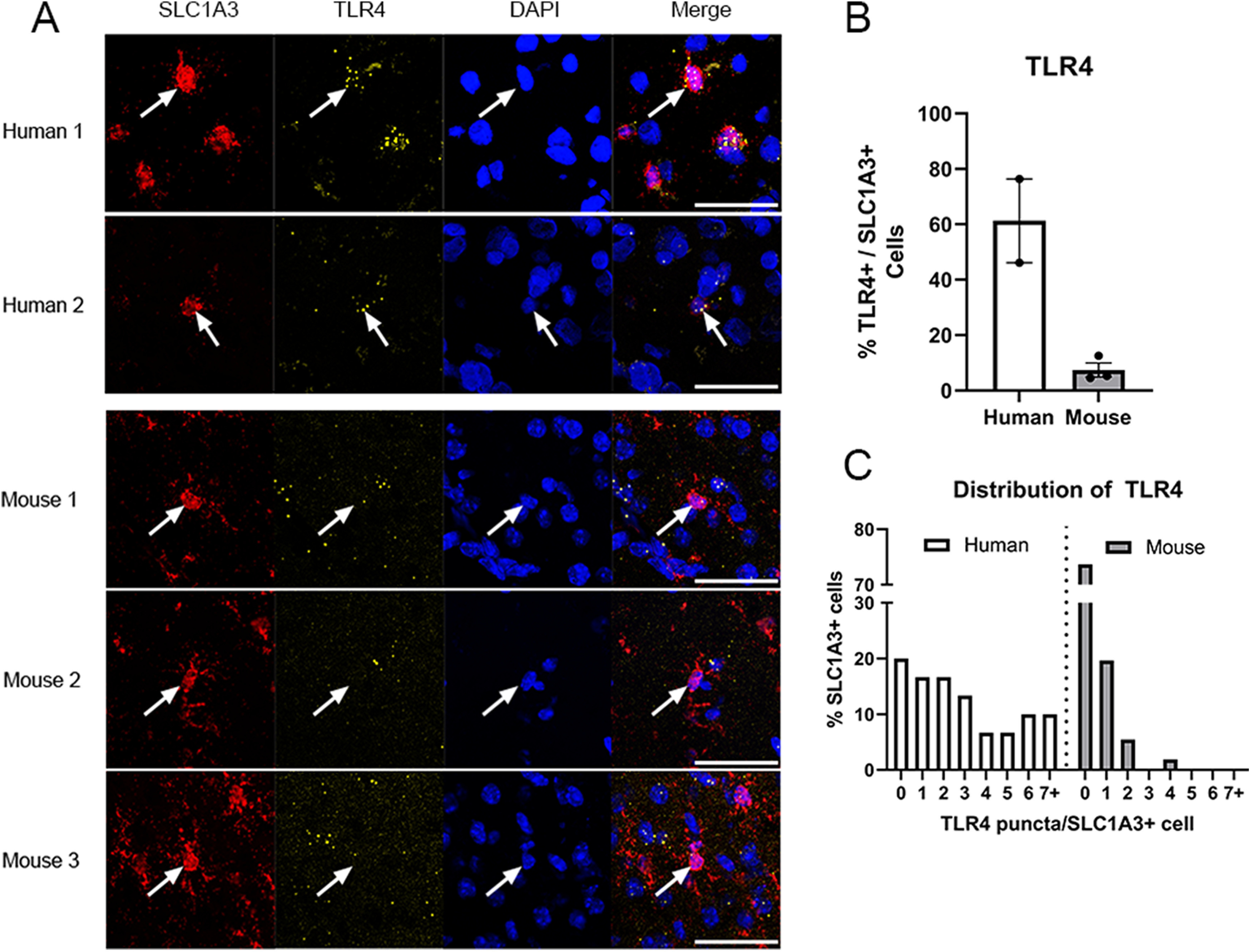
ISH validation of human astrocyte RNAseq. A, RNAscope ISH of TLR4 (yellow) in astrocytes (SLC1A3, red) in both human and mouse tissues. Scale bar, 50 μm. B, Quantification of TLR4+ astrocytes in human and mouse cortical tissue. Error bars indicate standard error. C, Histogram represents the number of TLR4+ puncta in an astrocytic cell body labeled by SLC1A3 in human and mouse cortical tissue.
The transcriptomes of astrocytes in FCD and control do not differ
Next, we examined the transcriptional signature of FCD. FCD is characterized by abnormalities in neuronal migration during development. Patients with FCD display abnormal radial and/or tangential lamination in a local region of cerebral cortex. More severe cases include dysmorphic neurons, and others also develop large and often multinucleated cells called balloon cells (Gaitanis and Donahue, 2013). Our DESeq2 analysis found only 24 protein-coding genes significantly associated with epilepsy, and all but one of those genes (SCN4B) had low expression (an average expression <1 RPKM; see Extended Data Fig. 4-1). Samples from FCD patients did not separate from controls in PCA, hierarchical clustering, or expression of reactive astrocyte markers (data not shown). Therefore, astrocytes in FCD in humans do not exhibit robust gene expression changes. However, we cannot exclude the possibility that a small subpopulation of astrocytes immediately adjacent to FCD lesions have gene expression changes that were undetectable at the population level. Given the lack of robust differences between control samples and epilepsy samples, they were used along with control samples in subsequent analyses.
Genes involved in ion transport and calcium signaling change with astrocyte maturation
Developing and mature brains have drastically different cognitive capacities, learning potentials, and susceptibilities to disease. Astrocytes are critical for the development of neural circuits, maintenance of homeostasis in adults, and responses and repair in neurologic diseases (Y. H. Huang et al., 2004; Sofroniew and Vinters, 2010; Chung et al., 2013). However, cellular and molecular changes of astrocytes during brain development and maturation in humans are unclear. Previous studies have performed transcriptome profiling of a small number of samples of human astrocytes from fetuses, children ≥8 years old, and adults (Zhang et al., 2016). The gene expression profiles of astrocytes during an important period of development (birth to 8 years) remain unknown. Therefore, molecular knowledge of astrocyte development and maturation in humans is incomplete. Here, we recruited patients throughout development and adulthood (n = 16 samples between 0 and 5 years old; 6 samples between 6 and 10 years old; 12 samples between 11 and 17 years old; and 8 adult samples, excluding peritumor; Extended Data Fig. 1-1), purified astrocytes, and performed RNA-seq. We analyzed maturation-associated genes based on our new RNAseq data using a linear model (DESeq2 R package, detailed in Materials and Methods) and also included fetal data from our previous study (Li et al., 2021) after normalizing data from two studies using percentiles (detailed in Materials and Methods). Our final results find 1509 upregulated genes and 1240 downregulated genes associated with astrocyte maturation across human development (Fig. 4C; Extended Data Fig. 4-2). Major astrocyte markers were not highly expressed in fetal astrocytes, but even the youngest postnatal samples (7 months) showed high expression of most markers. However, postnatal gene expression continued to evolve. Notably, top genes changing in the postnatal epoch displayed a shift in expression starting at ∼8 years old (Fig. 4A).
Figure 4.

Molecular characterization of human astrocyte maturation. Full maturation differential gene expression results are in Extended Data Figure 4-2, including FCD samples as they were not substantially different from controls (Extended Data Fig. 4-1). A, Heatmap of representative genes with changing expression across maturation (7 months to 21 years old, n = 26). Astrocytic gene expression approaches the mature pattern at ∼8 years of age. Plotted as Z score of gene expression (RPKM). Top bar represents rainbow index of sample ages. B, Selected GO terms enriched in genes that are upregulated (left) or downregulated (right) across maturation in both human astrocytes (black bars) and mouse astrocytes (gray bars) (Clarke et al., 2018). Dashed lines indicate p = 0.05. Full results are reported in Extended Data Figure 4-3. C, Venn diagrams quantifying astrocyte maturation-associated genes that are upregulated (left) and downregulated (right) in humans (red) and mice (blue). D, Top, Heatmap of selected astrocyte maturation markers colored by percentile change of RNA expression (e.g., Δ percentile = 100 demonstrates that a gene went from the least expressed gene to the most expressed gene) from fetal human astrocytes (Li et al., 2021) to mature human astrocytes (13-21 years old). Bottom, Heatmap of selected calcium signaling genes, same quantification as the heatmap above.
Differential Gene Expression in FCD Astrocytes. Download Figure 4-1, XLSX file (14.6KB, xlsx) .
Genes Upregulated Across Maturation. Download Figure 4-2, XLSX file (84.2KB, xlsx) .
GO Analysis of Maturation Genes; Human Up. Download Figure 4-3, XLSX file (135.7KB, xlsx) .
To assess the changes associated with astrocyte maturation, we analyzed GO of upregulated and downregulated astrocyte maturation genes using Metascape (see Extended Data Fig. 4-3). Upregulated genes showed significant enrichment for several GO terms related to ion homeostasis, as well as lipid metabolism (Fig. 4B). Many of the genes pertaining to ion transport are specifically related to calcium transport and signaling (Fig. 4D), which is intriguing given the importance of calcium as a signaling molecule, particularly in astrocytes. Therefore, immature and mature astrocytes may differ in ion transport and calcium signaling, thus altering many downstream signaling pathways that affect both astrocytes and surrounding neurons in development. Downregulated GO terms are almost entirely related to cell cycle and cell division, which is expected to decline throughout development (Fig. 4B). We also observe a general upward trend for several astrocyte marker genes that we would also expect to increase across maturation (Fig. 4D).
Characterizing the maturation of human astrocytes directly contributes to our understanding of human astrocyte biology and brain development, but most existing knowledge is derived from animal studies. Therefore, it is vital to determine which aspects of human biology are recapitulated by animal models and which are wholly unique. We compared our analysis of human astrocyte maturation with a published study that measured mouse astrocyte gene expression across several ages (Clarke et al., 2018). We compared astrocyte gene expression data from mice at an early developmental age, postnatal day 7 (P7), and a young adult time point, 10 weeks, and identified 4417 genes associated with mouse astrocyte maturation. Most of the mouse and human astrocyte maturation genes we found were not direct orthologs (Fig. 4C), and yet both gene lists had remarkably similar patterns based on GO (Fig. 4B). Both species downregulate cell division and upregulate ionic transport and calcium signaling genes across maturation. The conservation of these patterns in evolution suggests the importance of these astrocytic developmental changes. Based on this analysis, we find that human and mouse astrocytes share broad outcomes in maturation but differences concerning the exact pattern of molecular changes. While mouse models may not recapitulate every aspect of human biology, our data suggest that the maturation of astrocytes in humans can be accurately modeled in mice.
Human astrocytes downregulate genes involved in synaptic function in aging
Aging is associated with increased risk of cognitive decline and increased susceptibility to neurodegeneration and stroke. Astrocytes are important for maintaining homeostasis of the brain. Yet, aging-associated changes in human astrocytes are largely unknown. Characterizing these changes is the first step in elucidating potential involvement of astrocytes in aging-associated cognitive decline and neurodegeneration and developing astrocyte-targeted treatments. To identify genes with aging-associated expression, we began with genes significantly associated with age in the DESeq2 analysis of all samples that we also used to identify disease-related genes. To identify genes specifically associated with aging (changes after completing maturation) rather than general age (changes across the entire lifespan), we grouped samples into groups by age: 0-20 years old; 21-50 years old; and 50+ years old. From our list of age-associated genes, we extracted genes with average expression >1.5× higher or lower in the 50+ group versus the 21-50 group. We further filtered by minimum RPKM level of 0.01 to exclude lowly expressed genes. Thus, we identified 394 (277 protein-coding) genes significantly associated with aging (Extended Data Fig. 5-1).
As in peritumor astrocytes, we find decreased expression of genes mediating astrocyte-synaptic interactions in older astrocytes (Fig. 5A). Most notably, there is a reduction of SLC1A3, a glutamate transporter that clears glutamate from the extracellular space. Under normal conditions, SLC1A3 is a highly expressed core marker of adult astrocytes (Zhang et al., 2016). There is also a decline in CHRDL1, which codes for an astrocyte-secreted factor that drives synapse maturation (Blanco-Suarez et al., 2018). Aging astrocytes also have lower expression of two genes coding for glycoproteins found in the extracellular matrix. The first, CSPG5, shapes neurite growth and localizes around GABAergic and glutamatergic synaptic terminals (Pinter et al., 2020), while the second, OLMF1, binds synaptic proteins, such as synaptophysin and AMPARs (Nakaya et al., 2013). Declining expression of synaptic genes raises important questions about astrocytic roles in age-related cognitive decline and neurologic disease.
Figure 5.
Age-associated genes in human astrocytes. Full expression data are presented in Extended Data Figure 5-1. A, Expression of age-associated human astrocyte genes with decreased expression in older adults (50+ years old) compared with younger adults (21-50 years old). Error bars indicate standard error. B, Age-associated human astrocyte genes with increased expression in older adults compared with younger adults. All genes shown are significantly associated with age, and at least 1.5-fold enriched in younger or older adults. Error bars indicate standard error. C, Change in RNA expression of mouse astrocytes (10 weeks old vs 2 years old) (Clarke et al., 2018) in various GO categories. Gene lists derived from the following GO annotations, from left to right: GO:0046034, GO:0045202, GO:0019221, and GO:0090398. D, Protein–protein interaction networks among human (top) and mouse (bottom) age-associated genes.
Age-Associated Genes in Human Astrocytes. Download Figure 5-1, XLSX file (53.5KB, xlsx) .
Subjects over 50 show additional decreases in genes associated with energy metabolism (Fig. 5A). These include genes involved in mitochondrial generation of ATP, such as ATP5A1, an ATP-synthase subunit, MRPL35, a mitochondrial ribosomal component, and SLC25A5, a transporter that carries ATP out of the mitochondria. We also observe lower expression of the glycolytic enzyme PGAM1, and SLC13A5, a citrate transporter. Astrocytes are known to secrete citrate into the extracellular space where citrate has the ability to chelate calcium and magnesium ions, which are important to neuronal NMDA signaling (Westergaard et al., 2017). Together, these gene expression changes are consistent with decreased production of ATP in aging human astrocytes.
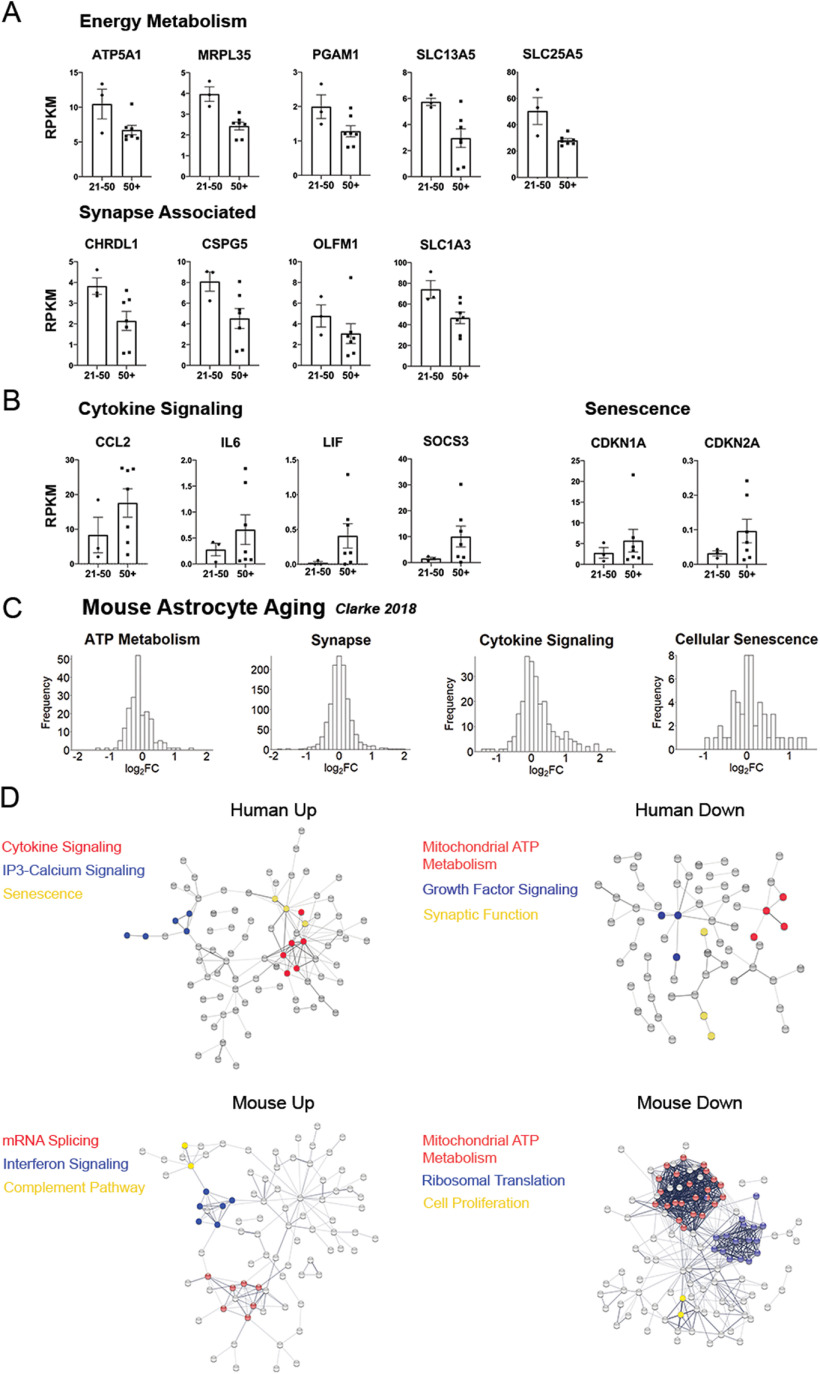
Last, we also observe an increase of several genes involved in cytokine signaling and senescence. These include the cytokines LIF, IL6, and CCL2, as well as cytokine regulator SOCS3 (Fig. 5B), which are also found in reactive astrocytes in mice (Zamanian et al., 2012). Therefore, these changes suggest that aging astrocytes may exhibit differences in interactions with neuronal synapses, altered energy metabolism, and increased cytokine signaling.
To assess the similarity between human and mouse astrocyte aging, we returned to the mouse aging dataset from Clarke et al. (2018). They reported a list of 58 age-associated genes in mouse cortical astrocytes. We wanted to determine whether mice recapitulated human changes related to metabolism, synapses, cytokines, and senescence. Because of the short length of the mouse gene list, we sought to identify trends in the whole dataset. To do so, we first chose relevant GO terms that captured the trends we observed in humans (“ATP metabolic process,” “synapse,” “cytokine-mediated signaling pathway,” and “cellular senescence”). Using these gene lists, we plotted differences in gene expression of all genes between the 10-week-old and 2-year-old mice (Fig. 5C). There is a prominent right skew in cytokine signaling genes, and we observe a slight left shift in ATP metabolism genes, suggesting that mouse astrocytes also show signs of upregulating cytokine signaling genes while downregulating metabolic genes in old age. The distributions for synaptic and senescence genes were highly symmetrical, suggesting no broad trends associated with age. However, mouse astrocytes could show important changes in smaller subsets of synaptic or senescence genes on further analysis. In total, we see evidence that mouse astrocytes share metabolic and cytokine features of human astrocyte aging.
Next, we examined changes in protein–protein interactions in aging astrocytes from humans and mice using the online tool STRING (www.string-db.org). We combined differentially expressed genes from three different studies of aging mouse astrocytes (Boisvert et al., 2018; Clarke et al., 2018; Ximerakis et al., 2019). Again, we see downregulation of mitochondrial ATP metabolism genes in both species, and both species also upregulated genes involved in inflammation, such as cytokine signaling, the complement pathway, and interferon response pathway (Fig. 5D). Furthermore, in aging human astrocytes, inositol triphosphate-calcium signaling pathway and senescence genes are upregulated, whereas growth factor signaling genes are downregulated. In aging mouse astrocytes, mRNA splicing genes are upregulated and ribosomal translation genes are downregulated.
Region-specific gene signatures in astrocytes
Studies in mice have found regional differences in gene expression, but little is known about potential differences across the human brain. We compared expression in temporal lobe (n = 27) and frontal lobe samples (n = 8) and found 64 differentially expressed genes. Interestingly, SEMA3A, a gene expressed at higher levels in ventral than in dorsal spinal cord astrocytes and repels axons from ventral spinal cords in mice (Molofsky et al., 2014), is expressed at higher levels in the frontal lobe than in the temporal lobe in humans, suggesting potentially conserved roles of astrocytic SEMA3A in region-specific axon guidance.
Subtle sexual dimorphism in astrocyte gene signatures
Female and male brains differ in their susceptibility to neurologic disorders. For example, intellectual disability, autism spectrum disorder, and Parkinson disease are more prevalent in men than in women (Werling and Geschwind, 2013; Gillies et al., 2014), whereas multiple sclerosis, Alzheimer disease, and anxiety disorder are more prevalent in women than in men (Seshadri et al., 1997; McLean et al., 2011; Westerlind et al., 2014). The cellular and molecular mechanisms underlying sexually dimorphic susceptibility to neurologic and psychiatric disorders are largely unknown. While understanding of sexually dimorphic properties of other brain cells, particularly microglia, is increasing, sexual dimorphism in human astrocytes has not yet been reported. In our differential gene expression analysis of all 49 samples, we found 105 genes (40 protein coding) with expression levels significantly associated with sex (Fig. 6; Extended Data Fig. 6-1). This gene list represents the first evidence of sexual dimorphism in human cortical astrocytes, to the best of our knowledge. Some of these genes are transcription factors (POU5F1B, HOXC10) or epigenetic factors, which may globally regulate gene expression. For example, the lysine demethylases KDM6A and KDM5C located on the X-chromosome are expressed at higher levels by female than male astrocytes. Females have two copies of X-chromosome genes, and males only have one copy. Most X-chromosome genes are subjected to X-inactivation in females, where only one copy of the gene is expressed, thus making expression levels comparable in males and females. Interestingly, KDM6A and KDM5C expression in female astrocytes is approximately twice as high as in male astrocytes, likely by escaping from X-inactivation, as has been reported in other cell types (Tricarico et al., 2020). KDM6A demethylates histone 3 lysine 27 trimethylation, a repressive mark found in promoters and enhancers, thus contributing to gene activation. KDM5C demethylates histone 3 lysine 4 methylation associated with active promoters and enhancers, thus contributing to gene repression. Both KDM6A and KDM5C are associated with risk of intellectual disability, a disease more common in males than in females (Zablotsky et al., 2017). Lower expression of these genes in male astrocytes may make them more susceptible to mutations that reduce demethylase activity, leading to astrocyte gene regulation defects, neural circuit dysfunction, and intellectual disability. We also observed a diverse array of differentially expressed genes whose protein products are located in the plasma membrane, although their functions in astrocytes remain mysterious (TMEM176B, TMEM143, CD99). Together, these data represent the first evidence that human astrocytes display a subtle sexual dimorphism at the molecular level.
Figure 6.
Sexually dimorphic genes in human astrocytes. Selected genes that are significantly associated with sex, including genes encoding transcription factors, epigenetic modifying enzymes, and proteins localized to the plasma membrane. Error bars indicate standard error. Full differential gene expression results are presented in Extended Data Figure 6-1.
Differential Gene Expression by Sex. Download Figure 6-1, XLSX file (2.2MB, xlsx) .
Discussion
We generated transcriptomic data of over 40 samples of acutely purified human astrocytes. These samples vary in age, sex, and disease state, allowing us to analyze these features in humans for the first time with RNAseq. Overall, genes associated with synaptic function change in multiple conditions, highlighting dynamism in astrocyte–synapse interactions in humans. Together, these data elucidate several fundamental aspects of human astrocyte biology in health and disease, as well as drawing important comparisons to murine astrocytes.
Molecular profile of astrocytes in human disease
Before the advent of the immunopanning technique, astrocyte purification mainly relied on serum selection (McCarthy and de Vellis, 1980). In these methods, heterogeneous collections of cells were cultured with serum-containing media that preferentially allowed survival and propagation of astrocytes. However, these conditions were not physiological, as serum is a component of the blood that does not cross the blood–brain barrier in healthy brain tissue. Astrocytes placed under these conditions upregulate reactive markers and adopt fibroblast-like morphology in culture (Foo et al., 2011; Zamanian et al., 2012). Using this method, it was challenging to study in vivo reactive astrocyte states, as the signal was masked by the response to serum during in vitro purification. Immunopanning allows for acute purification without the use of cell culture or serum, maintaining astrocytes in a near-physiological state (Zhang et al., 2016). This technique allowed us to characterize the transcriptomic profile of human astrocytes from two in vivo neurologic disorders, FCD, and brain tumor.
Despite previous evidence that some forms of epilepsy can induce astrocyte reactivity (Binder and Steinhauser, 2006), our analysis does not find notable changes in the astrocytes in FCD. This may reflect differences in disease progression across different kinds of epilepsy. A human study of patients with FCD only observed astrocyte reactivity in the center of the disorganized cortex, not in outer regions with milder neuronal phenotypes (Rossini et al., 2017). Therefore, it is conceivable that human astrocytes in this epileptic context would not necessarily demonstrate reactivity, and our findings in FCD should not be generalized to all forms of epilepsy. Additionally, bulk RNA-seq may miss reactive changes of a small subset of astrocytes.
In stark contrast, peritumor astrocytes demonstrate a robust change in gene expression. Peritumor astrocytes strongly decrease expression of glutamate transporters (SLC1A2 and SLC1A3) that are normally highly expressed in astrocytes and help maintain the excitation-inhibition balance of the brain. Seizure activity is common in individuals with brain tumors, and seizures are often the precipitating event that leads to medical treatment (Englot et al., 2016). Astrocytes may contribute to unregulated excitation in the brain by downregulating these important glutamate transporters. Excessive excitation not only causes harmful seizures; there is recent evidence that neurons form synaptic structures with tumor cells, and neuronal activity drives further proliferation and infiltration of tumor cells (Venkataramani et al., 2019; Venkatesh et al., 2019; Zeng et al., 2019). This raises the exciting possibility of therapeutically targeting glutamate uptake in astrocytes, in addition to existing antiseizure medications. In future studies, it will be vital to determine whether these general patterns hold for the various classes and origins of brain tumors, and at what stage of disease progression they appear. Beyond glutamate transporters, peritumor astrocytes downregulate several other genes that normally support synaptic function, suggesting further impacts on circuit function.
Indeed, our findings portray a general loss of function in peritumor astrocytes. There is a strong upregulation of cellular proliferation markers in conjunction with near-universal downregulation of core astrocyte genes. We specifically identified a widespread reduction of astrocyte receptor genes, which may limit their ability to sense and respond to their environment.
Interestingly, there may be one important gain of function in peritumor astrocytes. A study of human astrocytes from tumor cores concluded that astrocytes contributed to an immune-suppressive environment that permitted tumor proliferation and infiltration. Tumor-core astrocytes showed changes in the JAK/STAT and interferon γ response as well as upregulation of the anti-inflammatory cytokine Il-10 (Heiland et al., 2019), which were not present in the peritumor astrocytes. On the contrary, peritumor astrocytes upregulate many pro-inflammatory genes and downregulate anti-inflammatory genes. Although loss of synaptic support from peritumor astrocytes may contribute to circuit disruption and tumor-associated seizure activity, an increase in pro-inflammatory signaling may play a vital role in containing tumor growth and migration by promoting an immune response.
Human astrocyte maturation
Human brain development proceeds through a cascade of complex and reciprocal interactions between several maturing cell types. For example, neurons largely fail to make functional synapses in the absence of astrocytes, and astrocytes lack morphologic complexity without the presence of neurons (Banker, 1980; Stogsdill et al., 2017). The developmental trajectory of most major brain cells has been described by the presence of cell-specific transcription factors that drive cells toward a specific fate, such as NEUROD1 in neurons and OLIG2 in oligodendrocytes. Although some important regulators have been identified, astrocyte development and maturation remain less well understood (Kang et al., 2012; Glasgow et al., 2014; Chaboub et al., 2016; Li et al., 2019). Previous work compared fetal astrocytes to postnatal astrocytes that helped identify markers of mature versus immature astrocytes, and here we extend that work by creating a developmental timeline across postnatal astrocyte maturation in humans. This provides some of the first insight into how human astrocytes mature past the early stages of development. We found a shift in astrocytic gene expression at ∼8 years old that persists into early adulthood. This time frame coincides with the onset of increased synaptic pruning in the cortex, as evidenced by a decline in cortical synaptic spine density beginning around puberty (Huttenlocher, 1979; Huttenlocher and Dabholkar, 1997; Petanjek et al., 2011). Based on these data, astrocytes adopt functions for the support and maintenance of synapses before birth, but they adopt new roles in synaptic remodeling during postnatal maturation. These data also provide new markers to use in untangling astrocytic gene networks and molecular mechanisms that related to increased synaptic pruning. Pathway analysis of astrocyte maturation genes identified downregulation of proliferative pathways and upregulation of pathways related to ion homeostasis and lipid metabolism. We also find these patterns of astrocyte maturation preserved in mice, although mice and humans showed divergent sets of maturation-related genes. This suggests that mice and human astrocytes share many aspects of their developmental arcs, although they may express different sets of genes to achieve the same functional goal. Future studies should aim to identify the signaling mechanisms that drive astrocyte maturation, as aberrations in astrocyte maturation could contribute to dysfunction in neural circuits and ultimately neurodevelopmental disorders.
Aging in human astrocytes
Astrocytes become reactive in age-related neurologic diseases, such as Alzheimer disease (Beach et al., 1989). It is important to determine whether reactivity is induced purely by disease progression or whether astrocyte reactivity occurs in the course of normal aging, which may further contribute to aspects of disease progression. We observe declining expression of synaptic genes, including the glutamate transporter gene SLC1A3. As we noted in peritumor astrocytes, altered expression of this gene product would impact the balance of excitation-inhibition in the brain and impair circuit function. Two separate animal studies have identified increasing expression of reactive markers across age in the mouse brain, both in the cortex and subcortical regions (Boisvert et al., 2018; Clarke et al., 2018). We find corresponding evidence in our analysis of aging human astrocytes where several genes involved in cytokine signaling are upregulated, including CCL2, IL6, and SOCS3. We also observe a modest increase in senescence markers CDKN1A and CDKN2A. As our cohort only extends to age 65, further study of astrocytes at more advanced ages could uncover much larger changes in the astrocyte transcriptome throughout the aging process. While reactive and senescence markers increase, we observe a decrease in genes related to energy metabolism. Astrocytes typically provide metabolic support to aid in proper neuronal function and signaling, so these changes may contribute to age-related declines in cognition. An important question for further examination is whether a decrease in neuronal support is reflective of astrocytic dysfunction or declining demand from neurons. Our study does not have a large cohort of patients in the aging adult group. Given the higher variability of human data caused by greater genetic and environmental diversity compared with laboratory mice, future studies with large sample sizes will further expand our understanding of astrocyte aging in humans.
Sexual dimorphism of human astrocytes
Many neurologic diseases have differences in incidence and prognosis depending on sex, but little is known about the mechanisms that underlie these differences. Recently, multiple findings identified sex differences in microglia, but sex differences in astrocytes remain elusive despite their extensive interactions with microglia. Slight differences in astrocyte number and morphology were reported in subcortical regions of the brain in rats, such as the amygdala (Mong and McCarthy, 2002; Johnson et al., 2008). Here, we report the first evidence of sexual dimorphism in human astrocytes, to the best of our knowledge. Female cortical astrocytes have higher transcription of several plasma membrane proteins, including somatostatin receptor SSTR2, a transcriptional target of p53, PERP, and transmembrane protein TMEM176B. We also observe differential expression of genes encoding epigenetic regulators located on sex chromosomes, such as demethylases KDM5C and KDM6A. Although healthy astrocytes demonstrate relatively few sex differences, further studies should investigate whether underlying differences in epigenetic state could contribute to sex-specific responses to insult or injury and ultimately underlie sex differences in neurologic disease.
Naturally, limited access to fresh brain tissue limits many studies of the human brain, including this one, and our findings are not an exhaustive list of changes in human astrocytes. Further studies are needed to clarify context-dependent expression in human astrocytes in other diseases and patient populations to identify astrocytic roles in human health and disease. Our discovery of changes in genes involved in synaptic function across multiple conditions in human astrocytes is an important step in that direction, and our dataset will provide valuable insight for further investigation of human biology and novel approaches for neurologic disease.
Footnotes
This work was supported by Achievement Rewards for College Scientists Foundation Los Angeles Founder Chapter and National Institute of Mental Health, National Institutes of Health Award T32MH073526 to M.C.K.; Neurosurgery Research and Education Foundation Kate Carney Family Young Clinician Investigator Award to A.C.W.; National Institute of Neurological Disorders and Stroke of the National Institute of Health R00NS089780 and R01NS109025; National Institute of Aging, National Institutes of Health R03AG065772; National Center for Advancing Translational Science UCLA CTSI Grant UL1TR001881; the W. M. Keck Foundation Junior Faculty Award; UCLA Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research (BSCRC) Innovation Award; UCLA Jonsson Comprehensive Cancer Center and BSCRC Ablon Scholars Program; and Friends of the Semel Institute for Neuroscience & Human Behavior Friends Scholar Award to Y.Z. We thank Michael Sofroniew, Baljit Khakh, and Jessica Rexach for advice; Shane Liddelow for setting up our data website; and Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research, UCLA BioSequencing Core Facility for services.
Y.Z. has consulted for Ono Pharmaceutical. All remaining authors declare no competing interest.
References
- Allen NJ, Bennett ML, Foo LC, Wang GX, Chakraborty C, Smith SJ, Barres BA (2012) Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature 486:410–414. 10.1038/nature11059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S, Pyl PT, Huber W (2015) HTSeq: a Python framework to work with high-throughput sequencing data. Bioinformatics 31:166–169. 10.1093/bioinformatics/btu638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G (2000) Gene ontology: tool for the unification of biology. Nat Genet 25:25–29. 10.1038/75556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballas N, Lioy DT, Grunseich C, Mandel G (2009) Non-cell autonomous influence of MeCP2-deficient glia on neuronal dendritic morphology. Nat Neurosci 12:311–317. 10.1038/nn.2275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banker GA (1980) Trophic interactions between astroglial cells and hippocampal neurons in culture. Science 209:809–810. 10.1126/science.7403847 [DOI] [PubMed] [Google Scholar]
- Bausch-Fluck D, Goldmann U, Müller S, van Oostrum M, Müller M, Schubert OT, Wollscheid B (2018) The in silico human surfaceome. Proc Natl Acad Sci USA 115:E10988–E10997. 10.1073/pnas.1808790115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach TG, Walker R, McGeer EG (1989) Patterns of gliosis in Alzheimer's disease and aging cerebrum. Glia 2:420–436. 10.1002/glia.440020605 [DOI] [PubMed] [Google Scholar]
- Binder DK, Steinhauser C (2006) Functional changes in astroglial cells in epilepsy. Glia 54:358–368. 10.1002/glia.20394 [DOI] [PubMed] [Google Scholar]
- Blanco-Suarez E, Liu TF, Kopelevich A, Allen NJ (2018) Astrocyte-secreted chordin-like 1 drives synapse maturation and limits plasticity by increasing synaptic GluA2 AMPA receptors. Neuron 100:1116–1132.e1113. 10.1016/j.neuron.2018.09.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisvert MM, Erikson GA, Shokhirev MN, Allen NJ (2018) The aging astrocyte transcriptome from multiple regions of the mouse brain. Cell Rep 22:269–285. 10.1016/j.celrep.2017.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burda JE, Bernstein AM, Sofroniew MV (2016) Astrocyte roles in traumatic brain injury. Exp Neurol 275:305–315. 10.1016/j.expneurol.2015.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon S, Ireland A, Mungall CJ, Shu S, Marshall B, Lewis S, AmiGO Hub; Web Presence Working Group (2009) AmiGO: online access to ontology and annotation data. Bioinformatics 25:288–289. 10.1093/bioinformatics/btn615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaboub LS, Manalo JM, Lee HK, Glasgow SM, Chen F, Kawasaki Y, Akiyama T, Kuo CT, Creighton CJ, Mohila CA, Deneen B (2016) Temporal profiling of astrocyte precursors reveals parallel roles for ASEF during development and after injury. J Neurosci 36:11904–11917. 10.1523/JNEUROSCI.1658-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai H, Diaz-Castro B, Shigetomi E, Monte E, Octeau JC, Yu X, Cohn W, Rajendran PS, Vondriska TM, Whitelegge JP, Coppola G, Khakh BS (2017) Neural circuit-specialized astrocytes: transcriptomic, proteomic, morphological, and functional evidence. Neuron 95:531–549.e539. 10.1016/j.neuron.2017.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Sugihara H, Kim J, Fu Z, Barak B, Sur M, Feng G, Han W (2016) Direct modulation of GFAP-expressing glia in the arcuate nucleus bi-directionally regulates feeding. Elife 5:e18716. 10.7554/eLife.18716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA (2005) Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 120:421–433. 10.1016/j.cell.2004.12.020 [DOI] [PubMed] [Google Scholar]
- Chung WS, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C, Joung J, Foo LC, Thompson A, Chen C, Smith SJ, Barres BA (2013) Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature 504:394–400. 10.1038/nature12776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke LE, Liddelow SA, Chakraborty C, Munch AE, Heiman M, Barres BA (2018) Normal aging induces A1-like astrocyte reactivity. Proc Natl Acad Sci USA 115:E1896–E1905. 10.1073/pnas.1800165115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Majo M, Koontz M, Rowitch D, Ullian EM (2020) An update on human astrocytes and their role in development and disease. Glia 68:685–704. 10.1002/glia.23771 [DOI] [PubMed] [Google Scholar]
- Diaz-Castro B, Bernstein AM, Coppola G, Sofroniew MV, Khakh BS (2021) Molecular and functional properties of cortical astrocytes during peripherally induced neuroinflammation. Cell Rep 36:109508. 10.1016/j.celrep.2021.109508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR (2013) STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29:15–21. 10.1093/bioinformatics/bts635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling C, Allen NJ (2018) Mice lacking glypican 4 display juvenile hyperactivity and adult social interaction deficits. Brain Plast 4:197–209. 10.3233/BPL-180079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englot DJ, Chang EF, Vecht CJ (2016) Epilepsy and brain tumors. Handb Clin Neurol 134:267–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhy-Tselnicker I, van Casteren AC, Lee A, Chang VT, Aricescu AR, Allen NJ (2017) Astrocyte-secreted glypican 4 regulates release of neuronal pentraxin 1 from axons to induce functional synapse formation. Neuron 96:428–445.e413. 10.1016/j.neuron.2017.09.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo LC, Allen NJ, Bushong EA, Ventura PB, Chung WS, Zhou L, Cahoy JD, Daneman R, Zong H, Ellisman MH, Barres BA (2011) Development of a method for the purification and culture of rodent astrocytes. Neuron 71:799–811. 10.1016/j.neuron.2011.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaitanis JN, Donahue J (2013) Focal cortical dysplasia. Pediatr Neurol 49:79–87. 10.1016/j.pediatrneurol.2012.12.024 [DOI] [PubMed] [Google Scholar]
- Gandal MJ, Haney JR, Parikshak NN, Leppa V, Ramaswami G, Hartl C, Schork AJ, Appadurai V, Buil A, Werge TM, Liu C, White KP, Horvath S, Geschwind DH, Sestan N, Vaccarino F, Gerstein M, Weissman S, Pochareddy S, State M, et al. (2018a) Shared molecular neuropathology across major psychiatric disorders parallels polygenic overlap. Science 359:693–697. 10.1126/science.aad6469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandal MJ, Zhang P, Hadjimichael E, Walker RL, Chen C, Liu S, Won H, van Bakel H, Varghese M, Wang Y, Shieh AW, Haney J, Parhami S, Belmont J, Kim M, Moran Losada P, Khan Z, Mleczko J, Xia Y, Dai R, et al. (2018b) Transcriptome-wide isoform-level dysregulation in ASD, schizophrenia, and bipolar disorder. Science 362:eaat8127. 10.1126/science.aat8127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gene Ontology Consortium (2021) The Gene Ontology resource: enriching a GOld mine. Nucleic Acids Res 49:D325–D334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies GE, Pienaar IS, Vohra S, Qamhawi Z (2014) Sex differences in Parkinson's disease. Front Neuroendocrinol 35:370–384. 10.1016/j.yfrne.2014.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow SM, Zhu W, Stolt CC, Huang TW, Chen F, LoTurco JJ, Neul JL, Wegner M, Mohila C, Deneen B (2014) Mutual antagonism between Sox10 and NFIA regulates diversification of glial lineages and glioma subtypes. Nat Neurosci 17:1322–1329. 10.1038/nn.3790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Lai L, Butchbach ME, Stockinger MP, Shan X, Bishop GA, Lin CL (2003) Increased expression of the glial glutamate transporter EAAT2 modulates excitotoxicity and delays the onset but not the outcome of ALS in mice. Hum Mol Genet 12:2519–2532. 10.1093/hmg/ddg267 [DOI] [PubMed] [Google Scholar]
- Halassa MM, Florian C, Fellin T, Munoz JR, Lee SY, Abel T, Haydon PG, Frank MG (2009) Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron 61:213–219. 10.1016/j.neuron.2008.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Chen M, Wang F, Windrem M, Wang S, Shanz S, Xu Q, Oberheim NA, Bekar L, Betstadt S, Silva AJ, Takano T, Goldman SA, Nedergaard M (2013) Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell 12:342–353. 10.1016/j.stem.2012.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiland DH, Ravi VM, Behringer SP, Frenking JH, Wurm J, Joseph K, Garrelfs NW, Strähle J, Heynckes S, Grauvogel J, Franco P, Mader I, Schneider M, Potthoff AL, Delev D, Hofmann UG, Fung C, Beck J, Sankowski R, Prinz M, et al. (2019) Tumor-associated reactive astrocytes aid the evolution of immunosuppressive environment in glioblastoma. Nat Commun 10:2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howland DS, Liu J, She Y, Goad B, Maragakis NJ, Kim B, Erickson J, Kulik J, DeVito L, Psaltis G, DeGennaro LJ, Cleveland DW, Rothstein JD (2002) Focal loss of the glutamate transporter EAAT2 in a transgenic rat model of SOD1 mutant-mediated amyotrophic lateral sclerosis (ALS). Proc Natl Acad Sci USA 99:1604–1609. 10.1073/pnas.032539299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang AY, Woo J, Sardar D, Lozzi B, Bosquez Huerta NA, Lin CJ, Felice D, Jain A, Paulucci-Holthauzen A, Deneen B (2020) Region-specific transcriptional control of astrocyte function oversees local circuit activities. Neuron 106:992–1008.e1009. 10.1016/j.neuron.2020.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YH, Sinha SR, Tanaka K, Rothstein JD, Bergles DE (2004) Astrocyte glutamate transporters regulate metabotropic glutamate receptor-mediated excitation of hippocampal interneurons. J Neurosci 24:4551–4559. 10.1523/JNEUROSCI.5217-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR (1979) Synaptic density in human frontal cortex: developmental changes and effects of aging. Brain Res 163:195–205. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS (1997) Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol 387:167–178. [DOI] [PubMed] [Google Scholar]
- John Lin CC, Yu K, Hatcher A, Huang TW, Lee HK, Carlson J, Weston MC, Chen F, Zhang Y, Zhu W, Mohila CA, Ahmed N, Patel AJ, Arenkiel BR, Noebels JL, Creighton CJ, Deneen B (2017) Identification of diverse astrocyte populations and their malignant analogs. Nat Neurosci 20:396–405. 10.1038/nn.4493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RT, Breedlove SM, Jordan CL (2008) Sex differences and laterality in astrocyte number and complexity in the adult rat medial amygdala. J Comp Neurol 511:599–609. 10.1002/cne.21859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang P, Lee HK, Glasgow SM, Finley M, Donti T, Gaber ZB, Graham BH, Foster AE, Novitch BG, Gronostajski RM, Deneen B (2012) Sox9 and NFIA coordinate a transcriptional regulatory cascade during the initiation of gliogenesis. Neuron 74:79–94. 10.1016/j.neuron.2012.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley KW, Nakao-Inoue H, Molofsky AV, Oldham MC (2018a) Variation among intact tissue samples reveals the core transcriptional features of human CNS cell classes. Nat Neurosci 21:1171–1184. 10.1038/s41593-018-0216-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley KW, Ben Haim L, Schirmer L, Tyzack GE, Tolman M, Miller JG, Tsai HH, Chang SM, Molofsky AV, Yang Y, Patani R, Lakatos A, Ullian EM, Rowitch DH (2018b) Kir4.1-dependent astrocyte-fast motor neuron interactions are required for peak strength. Neuron 98:306–319.e307. 10.1016/j.neuron.2018.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofuji P, Newman EA (2004) Potassium buffering in the central nervous system. Neuroscience 129:1043–1056. 10.1016/j.neuroscience.2004.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krencik R, Hokanson KC, Narayan AR, Dvornik J, Rooney GE, Rauen KA, Weiss LA, Rowitch DH, Ullian EM (2015) Dysregulation of astrocyte extracellular signaling in Costello syndrome. Sci Transl Med 7:286ra66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krencik R, Seo K, van Asperen JV, Basu N, Cvetkovic C, Barlas S, Chen R, Ludwig C, Wang C, Ward ME, Gan L, Horner PJ, Rowitch DH, Ullian EM (2017) Systematic three-dimensional coculture rapidly recapitulates interactions between human neurons and astrocytes. Stem Cell Reports 9:1745–1753. 10.1016/j.stemcr.2017.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucukdereli H, Allen NJ, Lee AT, Feng A, Ozlu MI, Conatser LM, Chakraborty C, Workman G, Weaver M, Sage EH, Barres BA, Eroglu C (2011) Control of excitatory CNS synaptogenesis by astrocyte-secreted proteins Hevin and SPARC. Proc Natl Acad Sci USA 108:E440–E449. 10.1073/pnas.1104977108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffler SW, Nicholls JG, Orkand RK (1966) Physiological properties of glial cells in the central nervous system of amphibia. J Neurophysiol 29:768–787. 10.1152/jn.1966.29.4.768 [DOI] [PubMed] [Google Scholar]
- Laug D, Huang TW, Huerta NA, Huang AY, Sardar D, Ortiz-Guzman J, Carlson JC, Arenkiel BR, Kuo CT, Mohila CA, Glasgow SM, Lee HK, Deneen B (2019) Nuclear factor I-A regulates diverse reactive astrocyte responses after CNS injury. J Clin Invest 129:4408–4418. 10.1172/JCI127492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Kim JY, Noh S, Lee H, Lee SY, Mun JY, Park H, Chung WS (2021) Astrocytes phagocytose adult hippocampal synapses for circuit homeostasis. Nature 590:612–617. 10.1038/s41586-020-03060-3 [DOI] [PubMed] [Google Scholar]
- Li J, Khankan RR, Caneda C, Godoy MI, Haney MS, Krawczyk MC, Bassik MC, Sloan SA, Zhang Y (2019) Astrocyte-to-astrocyte contact and a positive feedback loop of growth factor signaling regulate astrocyte maturation. Glia 67:1571–1597. 10.1002/glia.23630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Pan L, Pembroke WG, Rexach JE, Godoy MI, Condro MC, Alvarado AG, Harteni M, Chen YW, Stiles L, Chen AY, Wanner IB, Yang X, Goldman SA, Geschwind DH, Kornblum HI, Zhang Y (2021) Conservation and divergence of vulnerability and responses to stressors between human and mouse astrocytes. Nat Commun 12:3958. 10.1038/s41467-021-24232-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung WS, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B, et al. (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541:481–487. 10.1038/nature21029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lioy DT, Garg SK, Monaghan CE, Raber J, Foust KD, Kaspar BK, Hirrlinger PG, Kirchhoff F, Bissonnette JM, Ballas N, Mandel G (2011) A role for glia in the progression of Rett's syndrome. Nature 475:497–500. 10.1038/nature10214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:550. 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Stork T, Bergles DE, Freeman MR (2016) Neuromodulators signal through astrocytes to alter neural circuit activity and behaviour. Nature 539:428–432. 10.1038/nature20145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy KD, de Vellis J (1980) Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol 85:890–902. 10.1083/jcb.85.3.890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean CP, Asnaani A, Litz BT, Hofmann SG (2011) Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. J Psychiatr Res 45:1027–1035. 10.1016/j.jpsychires.2011.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SJ, Philips T, Kim N, Dastgheyb R, Chen Z, Hsieh YC, Daigle JG, Datta M, Chew J, Vidensky S, Pham JT, Hughes EG, Robinson MB, Sattler R, Tomer R, Suk JS, Bergles DE, Haughey N, Pletnikov M, Hanes J, et al. (2019) Molecularly defined cortical astroglia subpopulation modulates neurons via secretion of Norrin. Nat Neurosci 22:741–752. 10.1038/s41593-019-0366-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AV, Krenick R, Ullian E, Tsai H, Deneen B, Richardson WD, Barres BA, Rowitch DH (2012) Astrocytes and disease: a neurodevelopmental perspective. Genes Dev 26:891–907. 10.1101/gad.188326.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AV, Kelley KW, Tsai HH, Redmond SA, Chang SM, Madireddy L, Chan JR, Baranzini SE, Ullian EM, Rowitch DH (2014) Astrocyte-encoded positional cues maintain sensorimotor circuit integrity. Nature 509:189–194. 10.1038/nature13161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mong JA, McCarthy MM (2002) Ontogeny of sexually dimorphic astrocytes in the neonatal rat arcuate. Brain Res Dev Brain Res 139:151–158. 10.1016/S0165-3806(02)00541-2 [DOI] [PubMed] [Google Scholar]
- Morel L, Chiang MS, Higashimori H, Shoneye T, Iyer LK, Yelick J, Tai A, Yang Y (2017) Molecular and functional properties of regional astrocytes in the adult brain. J Neurosci 37:8706–8717. 10.1523/JNEUROSCI.3956-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Y, Bennett DV, Rubinov M, Narayan S, Yang CT, Tanimoto M, Mensh BD, Looger LL, Ahrens MB (2019) Glia accumulate evidence that actions are futile and suppress unsuccessful behavior. Cell 178:27–43.e19. 10.1016/j.cell.2019.05.050 [DOI] [PubMed] [Google Scholar]
- Nagai J, Rajbhandari AK, Gangwani MR, Hachisuka A, Coppola G, Masmanidis SC, Fanselow MS, Khakh BS (2019) Hyperactivity with disrupted attention by activation of an astrocyte synaptogenic cue. Cell 177:1280–1292.e1220. 10.1016/j.cell.2019.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai J, Yu X, Papouin T, Cheong E, Freeman MR, Monk KR, Hastings MH, Haydon PG, Rowitch D, Shaham S, Khakh BS (2021) Behaviorally consequential astrocytic regulation of neural circuits. Neuron 109:576–596. 10.1016/j.neuron.2020.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaya N, Sultana A, Munasinghe J, Cheng A, Mattson MP, Tomarev SI (2013) Deletion in the N-terminal half of olfactomedin 1 modifies its interaction with synaptic proteins and causes brain dystrophy and abnormal behavior in mice. Exp Neurol 250:205–218. 10.1016/j.expneurol.2013.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard M (1994) Direct signaling from astrocytes to neurons in cultures of mammalian brain cells. Science 263:1768–1771. 10.1126/science.8134839 [DOI] [PubMed] [Google Scholar]
- Ng FS, Sengupta S, Huang Y, Yu AM, You S, Roberts MA, Iyer LK, Yang Y, Jackson FR (2016) TRAP-seq profiling and RNAi-based genetic screens identify conserved glial genes required for adult Drosophila behavior. Front Mol Neurosci 9:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen PT, Dorman LC, Pan S, Vainchtein ID, Han RT, Nakao-Inoue H, Taloma SE, Barron JJ, Molofsky AB, Kheirbek MA, Molofsky AV (2020) Microglial remodeling of the extracellular matrix promotes synapse plasticity. Cell 182:388–403.e315. 10.1016/j.cell.2020.05.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberheim NA, Wang X, Goldman S, Nedergaard M (2006) Astrocytic complexity distinguishes the human brain. Trends Neurosci 29:547–553. 10.1016/j.tins.2006.08.004 [DOI] [PubMed] [Google Scholar]
- Oberheim NA, Takano T, Han X, He W, Lin JH, Wang F, Xu Q, Wyatt JD, Pilcher W, Ojemann JG, Ransom BR, Goldman SA, Nedergaard M (2009) Uniquely hominid features of adult human astrocytes. J Neurosci 29:3276–3287. 10.1523/JNEUROSCI.4707-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen ML, Sontheimer H (2008) Functional implications for Kir4.1 channels in glial biology: from K+ buffering to cell differentiation. J Neurochem 107:589–601. 10.1111/j.1471-4159.2008.05615.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panickar KS, Norenberg MD (2005) Astrocytes in cerebral ischemic injury: morphological and general considerations. Glia 50:287–298. 10.1002/glia.20181 [DOI] [PubMed] [Google Scholar]
- Papouin T, Dunphy JM, Tolman M, Dineley KT, Haydon PG (2017) Septal cholinergic neuromodulation tunes the astrocyte-dependent gating of hippocampal NMDA receptors to wakefulness. Neuron 94:840–854.e847. 10.1016/j.neuron.2017.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park BS, Lee JO (2013) Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp Mol Med 45:e66. 10.1038/emm.2013.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG (1994) Glutamate-mediated astrocyte-neuron signalling. Nature 369:744–747. 10.1038/369744a0 [DOI] [PubMed] [Google Scholar]
- Petanjek Z, Judaš M, Šimić G, Rašin MR, Uylings HB, Rakic P, Kostović I (2011) Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci USA 108:13281–13286. 10.1073/pnas.1105108108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinter A, Hevesi Z, Zahola P, Alpar A, Hanics J (2020) Chondroitin sulfate proteoglycan-5 forms perisynaptic matrix assemblies in the adult rat cortex. Cell Signal 74:109710. 10.1016/j.cellsig.2020.109710 [DOI] [PubMed] [Google Scholar]
- Poskanzer KE, Molofsky AV (2018) Dynamism of an astrocyte in vivo: perspectives on identity and function. Annu Rev Physiol 80:143–157. 10.1146/annurev-physiol-021317-121125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail DF, Joyce JA (2017) The microenvironmental landscape of brain tumors. Cancer Cell 31:326–341. 10.1016/j.ccell.2017.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raore B, Schniederjan M, Prabhu R, Brat DJ, Shu HK, Olson JJ (2011) Metastasis infiltration: an investigation of the postoperative brain-tumor interface. Int J Radiat Oncol Biol Phys 81:1075–1080. 10.1016/j.ijrobp.2010.07.034 [DOI] [PubMed] [Google Scholar]
- Reichold M, Zdebik AA, Lieberer E, Rapedius M, Schmidt K, Bandulik S, Sterner C, Tegtmeier I, Penton D, Baukrowitz T, Hulton SA, Witzgall R, Ben-Zeev B, Howie AJ, Kleta R, Bockenhauer D, Warth R (2010) KCNJ10 gene mutations causing EAST syndrome (epilepsy, ataxia, sensorineural deafness, and tubulopathy) disrupt channel function. Proc Natl Acad Sci USA 107:14490–14495. 10.1073/pnas.1003072107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robel S, Buckingham SC, Boni JL, Campbell SL, Danbolt NC, Riedemann T, Sutor B, Sontheimer H (2015) Reactive astrogliosis causes the development of spontaneous seizures. J Neurosci 35:3330–3345. 10.1523/JNEUROSCI.1574-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini L, Garbelli R, Gnatkovsky V, Didato G, Villani F, Spreafico R, Deleo F, Lo Russo G, Tringali G, Gozzo F, Tassi L, de Curtis M (2017) Seizure activity per se does not induce tissue damage markers in human neocortical focal epilepsy. Ann Neurol 82:331–341. 10.1002/ana.25005 [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF (1996) Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron 16:675–686. 10.1016/S0896-6273(00)80086-0 [DOI] [PubMed] [Google Scholar]
- Sattler R, Tyler B, Hoover B, Coddington LT, Recinos V, Hwang L, Brem H, Rothstein JD (2013) Increased expression of glutamate transporter GLT-1 in peritumoral tissue associated with prolonged survival and decreases in tumor growth in a rat model of experimental malignant glioma. J Neurosurg 119:878–886. 10.3171/2013.6.JNS122319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshadri S, Wolf PA, Beiser A, Au R, McNulty K, White R, D'Agostino RB (1997) Lifetime risk of dementia and Alzheimer's disease: the impact of mortality on risk estimates in the Framingham Study. Neurology 49:1498–1504. 10.1212/WNL.49.6.1498 [DOI] [PubMed] [Google Scholar]
- Singh SK, Stogsdill JA, Pulimood NS, Dingsdale H, Kim YH, Pilaz LJ, Kim IH, Manhaes AC, Rodrigues WS Jr, Pamukcu A, Enustun E, Ertuz Z, Scheiffele P, Soderling SH, Silver DL, Ji RR, Medina AE, Eroglu C (2016) Astrocytes assemble thalamocortical synapses by bridging NRX1alpha and NL1 via Hevin. Cell 164:183–196. 10.1016/j.cell.2015.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV (2020) Astrocyte reactivity: subtypes, states, and functions in CNS innate immunity. Trends Immunol 41:758–770. 10.1016/j.it.2020.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV, Vinters HV (2010) Astrocytes: biology and pathology. Acta Neuropathol 119:7–35. 10.1007/s00401-009-0619-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stogsdill JA, Ramirez J, Liu D, Kim YH, Baldwin KT, Enustun E, Ejikeme T, Ji RR, Eroglu C (2017) Astrocytic neuroligins control astrocyte morphogenesis and synaptogenesis. Nature 551:192–197. 10.1038/nature24638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T, Okuyama S, Kawashima N, Hori S, Takimoto M, Wada K (1997) Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science 276:1699–1702. 10.1126/science.276.5319.1699 [DOI] [PubMed] [Google Scholar]
- Tasdemir-Yilmaz OE, Freeman MR (2014) Astrocytes engage unique molecular programs to engulf pruned neuronal debris from distinct subsets of neurons. Genes Dev 28:20–33. 10.1101/gad.229518.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian GF, Azmi H, Takano T, Xu Q, Peng W, Lin J, Oberheim N, Lou N, Wang X, Zielke HR, Kang J, Nedergaard M (2005) An astrocytic basis of epilepsy. Nat Med 11:973–981. 10.1038/nm1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricarico R, Nicolas E, Hall MJ, Golemis EA (2020) X- and Y-linked chromatin-modifying genes as regulators of sex-specific cancer incidence and prognosis. Clin Cancer Res 26:5567–5578. 10.1158/1078-0432.CCR-20-1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HH, Li H, Fuentealba LC, Molofsky AV, Taveira-Marques R, Zhuang H, Tenney A, Murnen AT, Fancy SP, Merkle F, Kessaris N, Alvarez-Buylla A, Richardson WD, Rowitch DH (2012) Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science 337:358–362. 10.1126/science.1222381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullian EM, Sapperstein SK, Christopherson KS, Barres BA (2001) Control of synapse number by glia. Science 291:657–661. 10.1126/science.291.5504.657 [DOI] [PubMed] [Google Scholar]
- Ung K, Tepe B, Pekarek B, Arenkiel BR, Deneen B (2020) Parallel astrocyte calcium signaling modulates olfactory bulb responses. J Neurosci Res 98:1605–1618. 10.1002/jnr.24634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainchtein ID, Chin G, Cho FS, Kelley KW, Miller JG, Chien EC, Liddelow SA, Nguyen PT, Nakao-Inoue H, Dorman LC, Akil O, Joshita S, Barres BA, Paz JT, Molofsky AB, Molofsky AV (2018) Astrocyte-derived interleukin-33 promotes microglial synapse engulfment and neural circuit development. Science 359:1269–1273. 10.1126/science.aal3589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaure C, Liu Y (2014) A comparative review of toll-like receptor 4 expression and functionality in different animal species. Front Immunol 5:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkataramani V, Tanev DI, Strahle C, Studier-Fischer A, Fankhauser L, Kessler T, Körber C, Kardorff M, Ratliff M, Xie R, Horstmann H, Messer M, Paik SP, Knabbe J, Sahm F, Kurz FT, Acikgöz AA, Herrmannsdörfer F, Agarwal A, Bergles DE, et al. (2019) Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature 573:532–538. 10.1038/s41586-019-1564-x [DOI] [PubMed] [Google Scholar]
- Venkatesh HS, Morishita W, Geraghty AC, Silverbush D, Gillespie SM, Arzt M, Tam LT, Espenel C, Ponnuswami A, Ni L, Woo PJ, Taylor KR, Agarwal A, Regev A, Brang D, Vogel H, Hervey-Jumper S, Bergles DE, Suvà ML, Malenka RC, et al. (2019) Electrical and synaptic integration of glioma into neural circuits. Nature 573:539–545. 10.1038/s41586-019-1563-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng Q, Wang J, Wang J, He D, Cheng Z, Zhang F, Verma R, Xu L, Dong X, Liao Y, He X, Potter A, Zhang L, Zhao C, Xin M, Zhou Q, Aronow BJ, Blackshear PJ, Rich JN, He Q, et al. (2019) Single-cell transcriptomics uncovers glial progenitor diversity and cell fate determinants during development and gliomagenesis. Cell Stem Cell 24:707–723.e708. 10.1016/j.stem.2019.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werling DM, Geschwind DH (2013) Sex differences in autism spectrum disorders. Curr Opin Neurol 26:146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westergaard N, Waagepetersen HS, Belhage B, Schousboe A (2017) Citrate, a ubiquitous key metabolite with regulatory function in the CNS. Neurochem Res 42:1583–1588. 10.1007/s11064-016-2159-7 [DOI] [PubMed] [Google Scholar]
- Westerlind H, Bostrom I, Stawiarz L, Landtblom AM, Almqvist C, Hillert J (2014) New data identify an increasing sex ratio of multiple sclerosis in Sweden. Mult Scler 20:1578–1583. 10.1177/1352458514530021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windrem MS, Osipovitch M, Liu Z, Bates J, Chandler-Militello D, Zou L, Munir J, Schanz S, McCoy K, Miller RH, Wang S, Nedergaard M, Findling RL, Tesar PJ, Goldman SA (2017) Human iPSC glial mouse chimeras reveal glial contributions to schizophrenia. Cell Stem Cell 21:195–208. e196. 10.1016/j.stem.2017.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler J, Abisoye-Ogunniyan A, Metcalf KJ, Werb Z (2020) Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat Commun 11:5120. 10.1038/s41467-020-18794-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Janczyk PL, Zhang Y, Liu A, Shi X, Singh S, Facemire L, Kubow K, Li Z, Jia Y, Schafer D, Mandell JW, Abounader R, Li H (2020) A cytoskeleton regulator AVIL drives tumorigenesis in glioblastoma. Nat Commun 11:3457. 10.1038/s41467-020-17279-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ximerakis M, Lipnick SL, Innes BT, Simmons SK, Adiconis X, Dionne D, Mayweather BA, Nguyen L, Niziolek Z, Ozek C, Butty VL, Isserlin R, Buchanan SM, Levine SS, Regev A, Bader GD, Levin JZ, Rubin LL (2019) Single-cell transcriptomic profiling of the aging mouse brain. Nat Neurosci 22:1696–1708. 10.1038/s41593-019-0491-3 [DOI] [PubMed] [Google Scholar]
- Yamanaka K, Chun SJ, Boillee S, Fujimori-Tonou N, Yamashita H, Gutmann DH, Takahashi R, Misawa H, Cleveland DW (2008) Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci 11:251–253. 10.1038/nn2047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Gozen O, Watkins A, Lorenzini I, Lepore A, Gao Y, Vidensky S, Brennan J, Poulsen D, Won Park J, Li Jeon N, Robinson MB, Rothstein JD (2009) Presynaptic regulation of astroglial excitatory neurotransmitter transporter GLT1. Neuron 61:880–894. 10.1016/j.neuron.2009.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Clyne M, Khoury MJ, Gwinn M (2010) Phenopedia and Genopedia: disease-centered and gene-centered views of the evolving knowledge of human genetic associations. Bioinformatics 26:145–146. 10.1093/bioinformatics/btp618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Taylor AM, Nagai J, Golshani P, Evans CJ, Coppola G, Khakh BS (2018) Reducing astrocyte calcium signaling in vivo alters striatal microcircuits and causes repetitive behavior. Neuron 99:1170–1187.e1179. 10.1016/j.neuron.2018.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Nagai J, Marti-Solano M, Soto JS, Coppola G, Babu MM, Khakh BS (2020) Context-specific striatal astrocyte molecular responses are phenotypically exploitable. Neuron 108:1146–1162.e1110. 10.1016/j.neuron.2020.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zablotsky B, Black LI, Blumberg SJ (2017) NCHS Data Brief No. 291: Estimated prevalence of children with diagnosed developmental disabilities in the United States, 2014–2016. [PubMed] [Google Scholar]
- Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA (2012) Genomic analysis of reactive astrogliosis. J Neurosci 32:6391–6410. 10.1523/JNEUROSCI.6221-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Q, Michael IP, Zhang P, Saghafinia S, Knott G, Jiao W, McCabe BD, Galvan JA, Robinson HPC, Zlobec I, Ciriello G, Hanahan D (2019) Synaptic proximity enables NMDAR signalling to promote brain metastasis. Nature 573:526–531. 10.1038/s41586-019-1576-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Sloan SA, Clarke LE, Caneda C, Plaza CA, Blumenthal PD, Vogel H, Steinberg GK, Edwards MS, Li G, Duncan JA, Cheshier SH, Shuer LM, Chang EF, Grant GA, Gephart MG, Barres BA (2016) Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron 89:37–53. 10.1016/j.neuron.2015.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, Tanaseichuk O, Benner C, Chanda SK (2019) Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun 10:1523. 10.1038/s41467-019-09234-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Human astrocyte metadata. Download Figure 1-1, XLSX file (12.8KB, xlsx) .
Human Astrocyte Gene Expression (RPKM). Download Figure 1-2, XLSX file (26.7MB, xlsx) .
Differential Gene Expression in Peritumor Astrocytes. Download Figure 2-1, XLSX file (186.1KB, xlsx) .
Cross-Validation of Differential Gene Expression; Peritumor digital gene expression leave-one-out. Download Figure 2-2, XLSX file (367.3KB, xlsx) .
Differential Gene Expression by Brain Region (Temporal versus Frontal Cortex). Download Figure 2-3, XLSX file (14.5KB, xlsx) .
Associations of Disease-Linked Genes and Peritumor Astrocyte Genes. Download Figure 2-4, XLSX file (11.8KB, xlsx) .
Astrocyte Receptor Genes. Download Figure 2-5, XLSX file (14.9KB, xlsx) .
GO Pathways Upregulated in Peritumor Astrocytes. Download Figure 2-6, XLSX file (62.8KB, xlsx) .
Differential Gene Expression in FCD Astrocytes. Download Figure 4-1, XLSX file (14.6KB, xlsx) .
Genes Upregulated Across Maturation. Download Figure 4-2, XLSX file (84.2KB, xlsx) .
GO Analysis of Maturation Genes; Human Up. Download Figure 4-3, XLSX file (135.7KB, xlsx) .
Age-Associated Genes in Human Astrocytes. Download Figure 5-1, XLSX file (53.5KB, xlsx) .
Differential Gene Expression by Sex. Download Figure 6-1, XLSX file (2.2MB, xlsx) .