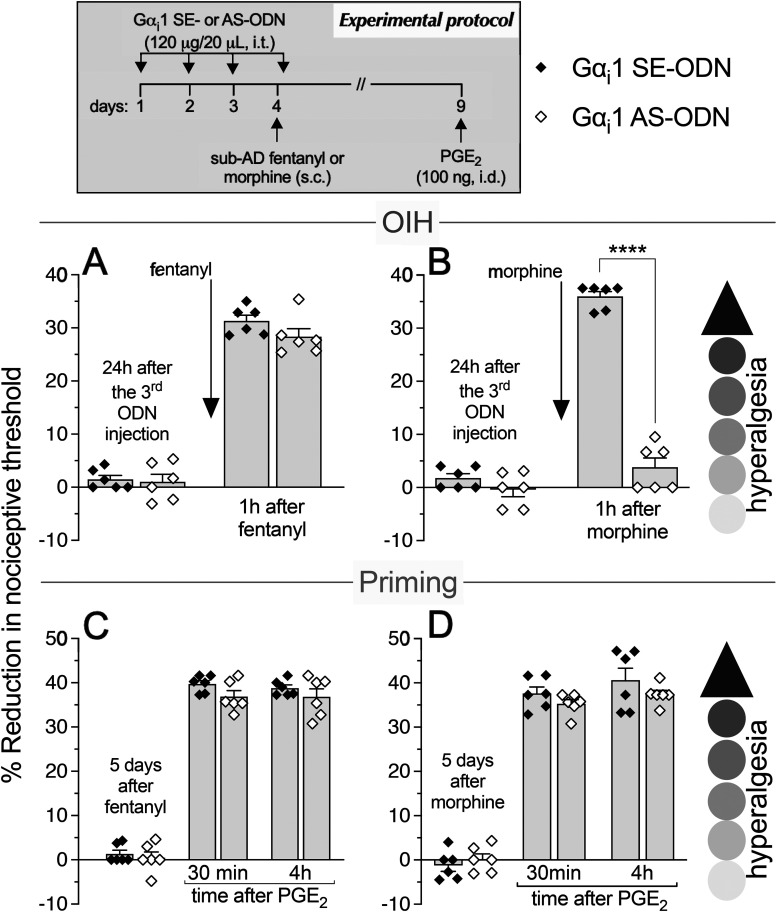
Figure 1.
Role of Gi-protein α1 subunit (Gαi1) in OIH and hyperalgesic priming produced by systemic sub-AD fentanyl and morphine. Rats received intrathecal (i.t.) injection of AS-ODN (120 μg/20 μl/d, i.t.) or SE-ODN (120 μg/20 μl/d, i.t.) to Gαi1 mRNA for 3 consecutive days. A, B, On the fourth day, at which time mechanical nociceptive threshold was not different from the pre-ODN baseline (A: SE-ODN-treated group: t(5) = 0.5; p = 0.64; AS-ODN-treated group: t(5) = 0.82; p = 0.45; B: SE-ODN-treated group: t(5) = 2.17; p = 0.08; AS-ODN-treated group: t(5) = 0.62; p = 0.56 when the mechanical nociceptive threshold is compared before and ∼17 h after the third ODN injection; paired Student's t test), sub-AD fentanyl (A: 0.01 mg/kg, s.c.) or morphine (B: 0.03 mg/kg, s.c.) was injected and the mechanical nociceptive threshold was evaluated 1 h later. Gαi1 AS-ODN did not affect sub-AD fentanyl-induced hyperalgesia (F(1,10) = 1.94, p = 0.19, when hyperalgesia was compared between the Gαi1 SE-ODN- and AS-ODN-treated groups 1 h after systemic sub-AD fentanyl; two-way repeated-measures ANOVA followed by Bonferroni post hoc test; A). However, in the Gαi1 AS-ODN-treated group, OIH produced by sub-AD morphine was markedly attenuated (F(1,10) = 189.4, ****p < 0.0001, when the hyperalgesia in the Gαi1 SE-ODN- and the AS-ODN-treated groups is compared at 1 h after systemic sub-AD morphine; two-way repeated-measures ANOVA followed by Bonferroni post hoc test; B). At the end of the fourth day, rats again received intrathecal Gαi1 AS- or SE-ODN. C, D, Five days after systemic sub-AD fentanyl and morphine, at which time mechanical nociceptive threshold was not different from preopioid baselines (C: SE-ODN-treated group: t(5) = 1.58; p = 0.18; AS-ODN-treated group that received sub-AD fentanyl: t(5) = 0.39; p = 0.71; D: SE-ODN-treated group: t(5) = 0.88; p = 0.42; AS-ODN-treated group that received sub-AD morphine: t(5) = 0.2; p = 0.85 when the mechanical nociceptive threshold is compared before and 5 d after systemic sub-AD opioid administration; paired Student's t test), PGE2 (100 ng/5 μl, i.d.) was injected and the mechanical nociceptive threshold was evaluated 30 min and 4 h later. Treatment with Gαi1 AS-ODN did not prevent PGE2-induced prolonged hyperalgesia in either fentanyl-treated (C) or morphine-treated (D) groups (C: F(1,10) = 2.12, p = 0.17; D: F(1,10) = 1.02, p = 0.34, when the hyperalgesia in the Gαi1 SE-ODN- and the AS-ODN-treated groups is compared at the fourth hour after intradermal PGE2 administration; two-way repeated-measures ANOVA followed by Bonferroni post hoc test). These findings support the suggestion that Gαi1 plays a role in OIH produced by systemic sub-AD morphine, but not fentanyl, and is not involved in hyperalgesic priming produced by sub-AD fentanyl or morphine (n = 6 paws/6 rats/group).