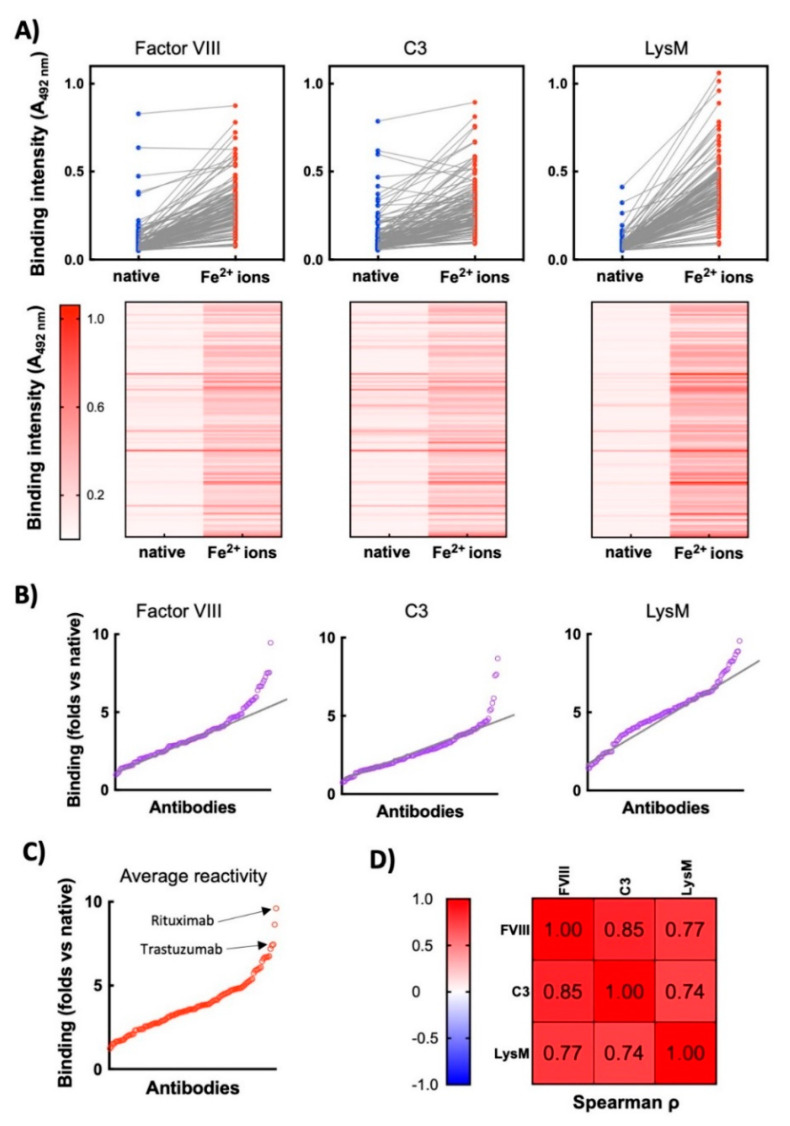
Figure 1.
Exposure of therapeutic Abs to ferrous ions results in induction of antigen-binding polyreactivity. (A) ELISA analyses of reactivity of 119 monoclonal therapeutic IgG1 Abs to immobilized proteins—human Factor VIII, human C3, and LysM from E. faecalis. Upper panels—the blue circles represent the reactivity of individual Abs in their native state; the red circles depict reactivity of Abs after exposure to ferrous ions. Each circle indicates the average binding intensity of a single Ab to a given antigen obtained from duplicate measurements. The reactivity of 113 of native Abs is taken from previous study [23]. Lower panels—heat maps showing the intensity of binding of each therapeutic Ab (in native form and after exposure to Fe2+ ions) to the indicated proteins. Reactivity of each Ab in the repertoire is represented as a horizontal line. Higher intensity of red indicates more substantial binding of Ab. (B) Fold increase in antigen-binding intensity after exposure to ferrous ions. The graphs show the ratio of reactivity of each Ab after exposure to Fe2+ ions versus reactivity of Ab in the native state towards Factor VIII, C3 and LysM. Each circle signifies one Ab. The graphs are generated from data presented in 1A. The black lines indicate the approximate trend in augmentation in the binding reactivity of repertoire towards indicated antigens. (C) Average increase in reactivity after exposure of therapeutic Abs to ferrous ions. The graph presents average values of fold increase binding of each Ab to the three unrelated antigens after exposure to ferrous ions. (D) Correlation analyses of Ab reactivities to different antigens. The table presents the correlation coefficients obtained after Spearman correlation analyses of binding reactivities of Fe2+-ion-treated Abs towards different antigens. All correlations are significant with p < 0.001.