FIGURE 4. High glucose reduces BK channel function and leads to a loss of ASL volume in CFBE cells in vitro.
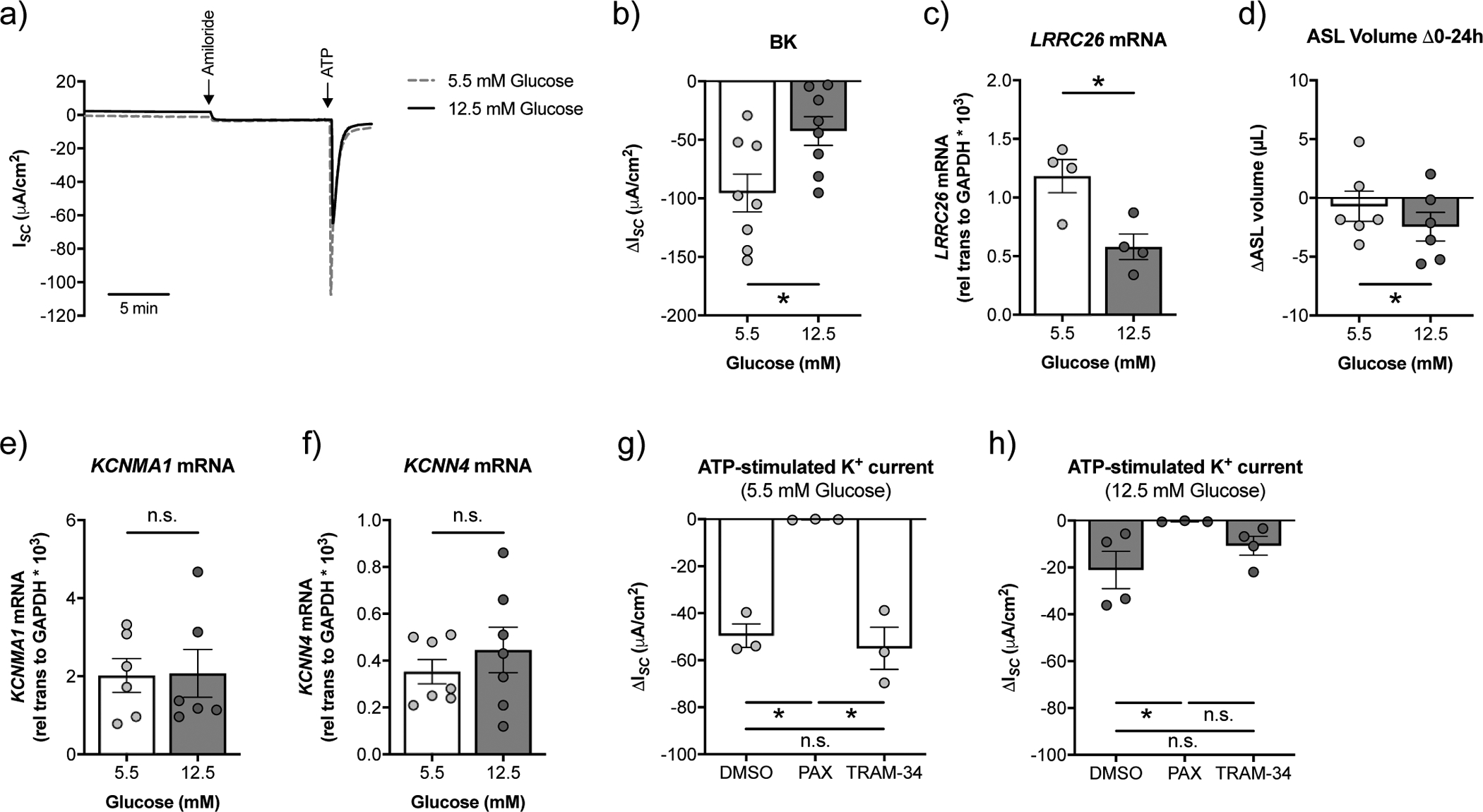
a) Representative tracing of ATP-stimulated ISC with a basolateral to apical K+ and apical to basolateral Na+ gradient [34] from fully differentiated CFBE cells cultured in ALI media with starting glucose concentrations of 5.5 mM or 12.5 mM. Ussing chamber experiments were performed on CFBE cells 20–24 h after basolateral media change. b) BK activity is significantly decreased in CFBE cells cultured under high glucose (24 h range: 10.1 – 12.5 mM) compared to normal glucose (24 range: 2.5 – 5.5 mM) (n=8 from 7 CF lungs). * p < 0.05, Student’s t-test. c) mRNA expression levels of LRRC26 inversely correlate with glucose levels (n=4 CF lungs). * p < 0.05, Student’s t-test. d) CFBE cells cultured under high glucose show increased ASL absorption (as indicated by a more negative ΔASL volume) over a 24 h period compared to CFBE cells under normal glucose (n=6 CF lungs). * p < 0.05, Student’s t-test. e) Expression of KCNMA1 mRNA is not significantly different in CFBE cells cultured in ALI media with starting glucose concentrations of 5.5 mM and 12.5 mM (n=6 from 6 CF lungs). n.s.=not significant, Wilcoxon test. f) The KCa3.1 potassium channel is encoded by KCNN4. High glucose does not affect the expression of KCNN4 mRNA in CFBE cells (n=7 from 7 CF lungs). n.s.=not significant, Wilcoxon test. g) ATP-stimulated K+ currents are significantly reduced by 10 μM paxilline (PAX), but not 1 μM TRAM-34, in CFBE cells cultured in ALI media with a starting glucose concentration of 5 mM (n=3 CF lungs). * p < 0.05, one-way ANOVA with Tukey’s. h) ATP-stimulated K+ currents are significantly reduced by 10 μM paxilline (PAX) in CFBE cells cultured in ALI media with a starting glucose concentration of 12.5 mM. The reduction in ATP-stimulated K+ currents by TRAM-34 was not significant (n ≥ 3 CF lungs). * p < 0.05, Kruskal-Wallis test with Dunn’s. n.s.=not significant. Data are shown as mean ± S.E.M.
