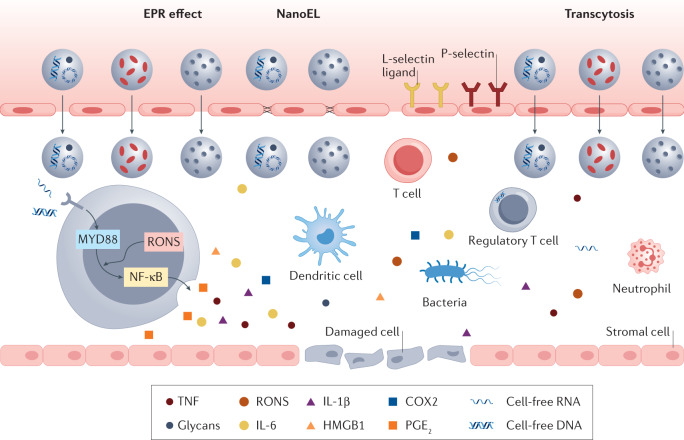
Fig. 2. The inflammatory microenvironment.
The inflammatory microenvironment comprises invasive pathogens, damaged cells and vasculature, infiltrating immune cells, danger signals, such as pathogen-associated molecular patterns and endogenous tissue damage-associated molecular patterns, and a plethora of pro-inflammatory molecules, such as cytokines, chemokines, enzymes, leukotrienes and eicosanoids. In particular, reactive oxygen and nitrogen species (RONS) can activate Toll-like-receptor-mediated nuclear factor-κB (NF-κB) and interferon regulatory factor pathways during inflammation. Localized inflammation induces the activation of microvascular endothelial cells, causing changes in vascular permeability to promote leukocyte homing, such as neutrophil adhesion and transmigration, as well as activation of platelets and monocytes. Nanoparticles can accumulate in the inflammatory microenvironment owing to the enhanced permeability and retention (EPR) effect, transcytosis or nanomaterials-induced endothelial leakiness (NanoEL). COX2, cyclooxygenase 2; HMGB1, high-mobility group box 1; IL-1β, interleukin-1β; IL-6, interleukin-6; PGE2, prostaglandin E2; TNF, tumour necrosis factor.