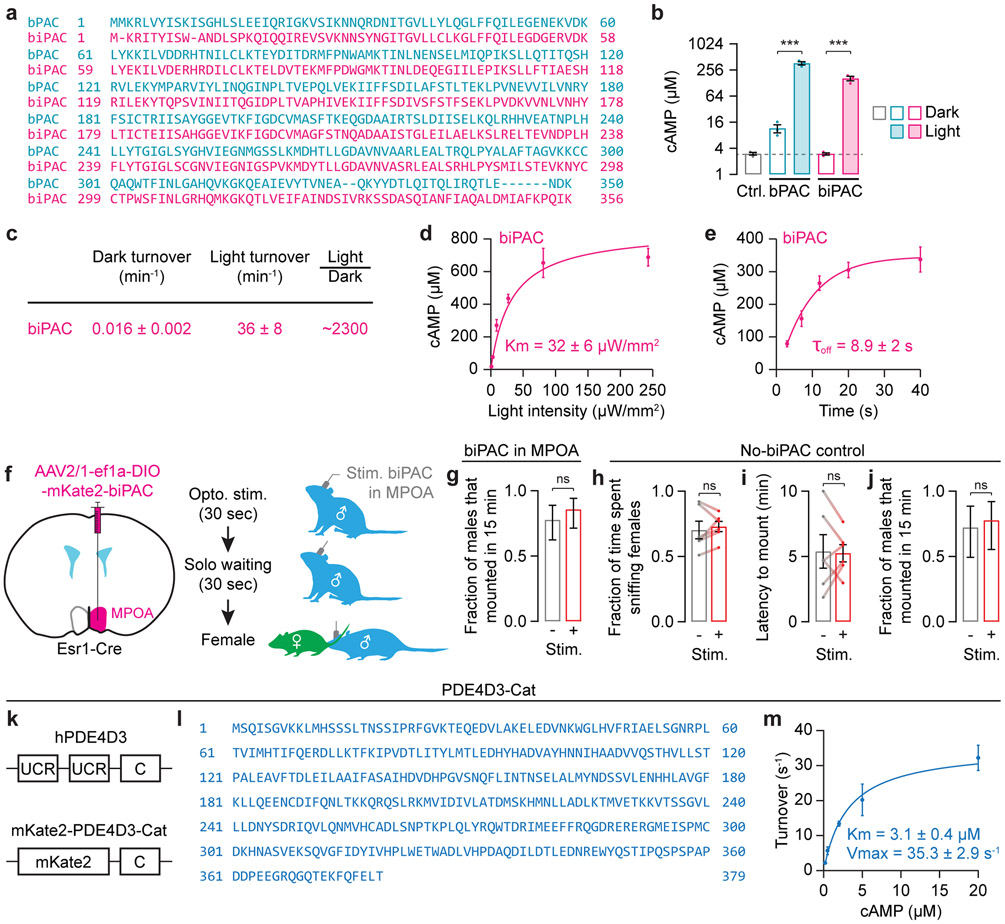
Extended Data Fig. 10 ∣. Novel optogenetic and molecular tools to manipulate intracellular cAMP in vivo.
a, Peptide sequences of bPAC63 and an improved variant, biPAC.
b, cAMP production by bPAC and biPAC in Xenopus laevis oocytes. Measurement was performed 3 days after injection of 30 ng cRNA of Venus-bPAC or Venus-biPAC in the dark (‘Dark’, non-shaded bars) or after 1 min illumination with 473 nm, 0.3 mW/mm2 light (‘Light’, shaded bars). The control bar shows uninjected oocytes (n = 3 groups of 5 oocytes). For comparison, the mean value of the uninjected oocytes is also noted with a horizontal dashed line. The Y-axis is on the log scale. Note that, in contrast to bPAC, biPAC does not exhibit increased enzymatic activity in darkness, above levels observed in baseline control conditions.
c, Enzymatic activity of Venus-biPAC in dark and light conditions (n = 4, mean ± s.d.).
d, Light intensity-dependent cAMP production of Venus-biPAC at 473 nm. After fitting with the Michaelis-Menten function, the Km value was determined to be 32 μW/mm2 (n = 4, mean ± s.d.). Venus tag was used here for protein-quantification purpose (same below).
e, cAMP concentrations at different time points in the dark after 500 ms of light stimulation. Fitting a mono-exponential function yielded an off time constant τoff = 8.9 ± 2.2 s (n = 4, mean ± s.d.).
f,g, Brief optogenetic stimulation of biPAC expressed in Esr1-Cre cells in the MPOA did not further increase the fraction of males that mount in the first 15 minutes after subsequent introduction of the female, as this fraction was already near ceiling in the absence of stimulation (ns, non-significant; Fisher’s exact test, n = 35, 36 trials from 12 males, mean ± 95% c.i.).
h-j, In mice that did not express biPAC, the same stimulation protocol failed to increase appetitive sniffing (h: n = 6 males), failed to decrease the latency to consummatory mounting behaviors (i: n = 6 males), and failed to increase the fraction of males that mounted (j: n = 18 trial from 6 males, mean ± 95% c.i.).
k,l, Design (k) and sequence (l) of PDE4D3-Cat. Upstream conserved regions (UCRs) are inhibitory, regulatory domains in the endogenous hPDE4D364. Replacement of these UCRs with mKate2 substantially increased the enzymatic activity of PDE4D3-Cat over hPDE4D3.
m, Enzymatic activity of Venus-PDE4D3-Cat (n = 3, mean ± s.d.). After fitting with the Michaelis-Menten function, the Km value was determined to be 3.1 ± 0.4 μM and the Vmax value was 35.3 ± 2.9 s−1. This enzyme is much faster than the endogenous protein (Vmax = 5-9 s−1)64.
***p<0.001. See Supplementary Table 1 for statistics.