Supplemental Digital Content is available in the text.
Keywords: aphasia, big data, comprehension, language therapy, meta-analysis, stroke
Background and Purpose:
Optimizing speech and language therapy (SLT) regimens for maximal aphasia recovery is a clinical research priority. We examined associations between SLT intensity (hours/week), dosage (total hours), frequency (days/week), duration (weeks), delivery (face to face, computer supported, individual tailoring, and home practice), content, and language outcomes for people with aphasia.
Methods:
Databases including MEDLINE and Embase were searched (inception to September 2015). Published, unpublished, and emerging trials including SLT and ≥10 individual participant data on aphasia, language outcomes, and time post-onset were selected. Patient-level data on stroke, language, SLT, and trial risk of bias were independently extracted. Outcome measurement scores were standardized. A statistical inferencing, one-stage, random effects, network meta-analysis approach filtered individual participant data into an optimal model examining SLT regimen for overall language, auditory comprehension, naming, and functional communication pre-post intervention gains, adjusting for a priori–defined covariates (age, sex, time poststroke, and baseline aphasia severity), reporting estimates of mean change scores (95% CI).
Results:
Data from 959 individual participant data (25 trials) were included. Greatest gains in overall language and comprehension were associated with >20 to 50 hours SLT dosage (18.37 [10.58–26.16] Western Aphasia Battery–Aphasia Quotient; 5.23 [1.51–8.95] Aachen Aphasia Test–Token Test). Greatest clinical overall language, functional communication, and comprehension gains were associated with 2 to 4 and 9+ SLT hours/week. Greatest clinical gains were associated with frequent SLT for overall language, functional communication (3–5+ days/week), and comprehension (4–5 days/week). Evidence of comprehension gains was absent for SLT ≤20 hours, <3 hours/week, and ≤3 days/week. Mixed receptive-expressive therapy, functionally tailored, with prescribed home practice was associated with the greatest overall gains. Relative variance was <30%. Risk of trial bias was low to moderate; low for meta-biases.
Conclusions:
Greatest language recovery was associated with frequent, functionally tailored, receptive-expressive SLT, with prescribed home practice at a greater intensity and duration than reports of usual clinical services internationally. These exploratory findings suggest critical therapeutic ranges, informing hypothesis-testing trials and tailoring of clinical services.
Registration:
URL: https://www.crd.york.ac.uk/PROSPERO/; Unique identifier: CRD42018110947.
Every year, an estimated 4.5 million stroke survivors world wide experience aphasia, resulting in difficulties with speaking, understanding speech (auditory comprehension), reading, writing, and communication.1,2 Despite accessing more rehabilitation resources than stroke survivors without aphasia,2,3 people with aphasia experience poorer functional outcomes,4 diminished social networks,5 and fewer return home or to work.6,7 Aphasia has major health, psychosocial, and economic impacts.
Aggregate meta-syntheses of 27 trials (n=1620) of speech and language therapy (SLT) for aphasia provided important evidence that people with aphasia benefit from SLT on measures of language production, comprehension, and functional communication, compared with trial participants with no access to therapy.8 Identifying optimal therapy regimens across individual randomized controlled trials (RCTs) delivering protocolized interventions at a precise intensity, duration, dosage, and frequency, targeting specific language change within a defined trial participant population is difficult. Intervention complexity, heterogeneity of aphasia and participant profiles, and the variability of outcome measures obscure comparisons, and individual trial outcomes may appear inconsistent.
Meta-analysis of 6 trials highlighted the benefit of intensive therapy regimens while raising importance of time poststroke and tolerance concerns.8 Aggregate data analysis approaches, however, hinder detailed examination of participant covariates.8,9,9a Ecological bias is a critical consideration in aggregate data meta-analyses where individual variability of treatment effect associations may be concealed. Research and clinical uncertainties about the optimal SLT regimens remain. Consequently, clinical service reports describe an average of 60 to 90 minutes of SLT weekly for patients within 3 months of aphasia onset10–15 (declining to 45 minutes monthly thereafter) and 4 to 16 hours total dosage.10
Examination of the optimal SLT regimen requires large individual participant data (IPD) meta-analyses. Previously, large aphasia data set investigations captured measures of language severity on generic stroke scales or screening tools, limiting clinical interpretation.2,16,17 The REhabilitation and recovery of peopLE with Aphasia after StrokE Collaboration created an international IPD aphasia database supporting a protocol-based network meta-analyses.18 We examined SLT intensity (hours/week), duration (weeks), dosage (total SLT hours), frequency (days/week), delivery (face to face, computer supported, or self-managed), setting, and provider associations with variations in language recovery gains from pre- to postintervention on measures of overall language, auditory comprehension, naming, and functional communication among people with aphasia after stroke.18
Methods
To minimize the possibility of unintentionally sharing information that can be used to reidentify private information and to ensure adherence to primary and meta-data set ethical approvals, a subset of the data generated for this study is available via the Collaboration of Aphasia Trialists www.aphasiatrials.org. We referred to the relevant PRISMA guidelines and extensions to support the reporting of this IPD network meta-analysis of complex interventions.19,20
Search Strategy and Selection
Our IPD network meta-analysis informed by an RCT-optimized systematic review (inception to September 2015 plus trial registrations for emerging trials) identified published and unpublished data sets with ≥10 IPD on aphasia, language outcome, and time since stroke.18 We systematically searched several electronic databases including MEDLINE and EMBASE, checking reference lists. We translated non-English data sets, extracted eligible public domain data sets, enquired about registered trial availability, and invited data set contributions including trials completed beyond the electronic search date.18 Thus, we sought IPD both directly from investigators in electronic format and where available in the public domain. Language information derived from screening measures or generic stroke scales was excluded.
Two independent reviewers considered full text reports. A third resolved disagreements. Potentially eligible primary data sets were invited to contribute data. Nonrespondents were sent one reminder, and coauthors were contacted. Respondents confirmed data set eligibility before IPD contribution. Data searching, identification, extraction, and analyses were guided by our published protocol (https://www.crd.york.ac.uk/PROSPERO/; CRD42018110947).18 Included data sets had relevant ethical and gatekeeper approvals. We secured university ethical approval (HLS/NCH/15/09) and UK national health service regulatory registration for our research and database (IRAS ID 179505).
Data Extraction and Preparation
We extracted IPD on demography (age, sex, living context, and language), stroke (time post-onset, type, lesion hemisphere, and severity at baseline), SLT intervention, and language outcome (overall language, auditory comprehension, naming, and functional communication raw scores). Language recovery was defined as change in absolute language score from baseline to first postintervention follow-up and collated outcome measurement instruments by language outcome, agreed a priori by the REhabilitation and recovery of peopLE with Aphasia after StrokE collaborators. We confirmed baseline and subsequent time point data extraction and sought unreported data from the primary researchers where possible.9a,18
For each language outcome, we identified the outcome measurement most frequently used by included data sets (anchor measure) and transformed the remainder (minority measures) to match the anchor’s range and format, thus retaining a clinically meaningful change from baseline score as a measure of effect size.18 Anchor measure overall language scores were represented by the Western Aphasia Battery–Aphasia Quotient (WAB-AQ), auditory comprehension was represented by the Aachen Aphasia Test–Token Test (AAT-TT), naming by the Boston Naming Test, and functional communication by the Aachen Aphasia Test–Spontaneous Speech Communication (AAT-SSC) rating score.21
Data on SLT interventions were categorized by regimen (frequency, intensity, duration, and dosage), content (home practice, theoretical approach, language target, individualized tailoring by functional relevance, or difficulty level), delivery (face to face, computer supported, or self-managed), setting (in/outpatient), and provider (professional/nonprofessional).18,21 We cross-checked data with primary research teams and available documentation. An independent researcher checked data extraction. Unavailable data were recorded as unreported. We excluded IPD where aphasia had a nonstroke etiology, time post-onset was unreported, and any IPD duplications. Protocolized intervention descriptions at group level were applied to IPD within each group accordingly. Where tailoring of interventions or home practice tasks were not reported, we assumed absence. Final data formatting decisions were made following discussion with the REhabilitation and recovery of peopLE with Aphasia after StrokE collaborators. Categorical formats (eg, 3–4 weeks) were recorded as mean (3.5 weeks). Pharmacological and neurostimulation cointerventions were documented. Crossover data sets were included up to crossover.18
Network Meta-Analysis
Network meta-analyses of SLT interventions and language outcomes were undertaken with data set as a random effect and demographics and interventions as fixed effects (SAS 9.4 using PROC MIXED).27 Our 1-stage network meta-analysis, incorporating prespecified potential confounders in the base model (age, sex, aphasia severity at study entry, and time post-onset), combined eligible IPD into a single model that considered clustering by data set.9a,18 Our statistical inferencing approach synthesized and examined data relating to SLT intervention and associations with language recovery gains.18,22,25 The minimum sample size for each network meta-analysis was 20 IPD (2 RCTs).
Each SLT intervention variable was considered simultaneously, and continuous SLT regimen variables were grouped (eg, 10 versus 50 hours dosage). We examined SLT intervention categories’ contribution to the base model, the magnitude of differences, and the intervention components’ stratum.18 For each language outcome, treatment effect was defined as the mean absolute change from baseline to the first follow-up after intervention on the transformed standardized measure. Emphasis was placed on reporting estimates of means and 95% CIs, from which the degree of certainty of the effect size could be evaluated.9a Clinically interesting differences were presented in addition to those that reached statistical significance, thus highlighting important considerations to be examined within future RCTs where therapy regimen and delivery might be optimally predefined.18,22
The impact of IPD and language variables on the intervention effect were examined simultaneously. We examined IPD clustering within RCTs, distinguishing IPD from data set–based interactions.9a Network graphs (generated using the GNU PSPP program) facilitated a review and summary of the network balance, highlighting isolated interventions with no networked comparators, which were excluded from analyses. Variance was unstructured, and data set variance was assessed.18 Analysis was restricted to available data. Where >20% of a data set variable was missing, it was excluded from that network analysis. We reviewed patterns of loss, compared missing data to demographic and other variables using the independent t test or Mann-Whitney U tests. Where there was no evidence of influence, the data were considered missing at random. Data not missing at random were excluded.18
Risk of Bias
We undertook rigorous quality-verification checks including sequence generation, to ensure data were valid, reliable, consistent, and as complete as possible.9a,18 Data set biases (selection, performance, detection, and attrition bias) were rated as low, unclear, or high risk9a,27. The risk of meta-biases (selection, publication, and availability bias) was also considered.28 Primary data set clinical, methodological, and statistical heterogeneity was considered. Methodological differences were recorded as risk of bias. Our data synthesis procedures accommodated between-study outcome differences.18
Each of our planned analyses were unique in participant, intervention, and outcome IPD, making standard heterogeneity assessments (I2) unsuitable. Instead, we compared variability due to study differences to data variability overall. Where variability was >25%, we checked data sets for undue influence or unbalanced groups; >50% variability was considered unreliable. Meta-analysis decisions were examined including the choice of measurements informing language outcomes, exclusion of minority measures, use of random rather than fixed effect,26 and inclusion of historical data sets (before 2000). We considered the quality of data sets, meta-syntheses, and impact on our findings. Our research grant funders had no role in the study design, data collection, analysis, interpretation, or writing of the report. The corresponding author had full data access and final responsibility for the decision to submit for publication.
Results
Studies Screened and Included
Of the 5276 records screened, we reviewed 1131 full texts, inviting 698 (including 193 trial registrations) to confirm eligibility and availability and to contribute data to support our planned analyses (Figure I in the Supplemental Material).21 We received IPD electronic contributions directly from trialists and extracted IPD from the public domain. Of the 174 data sets included, representing 5928 stroke survivors with aphasia, 91 included language interventions and 45 were RCTs. After filtering of data sets for availability of demographic, intervention, and relevant language data items, 25 RCTs (928 IPD)24,29–52 informed our planned network meta-analyses (Figure I in the Supplemental Material; Tables I through III in the Supplemental Material).
Study Characteristics
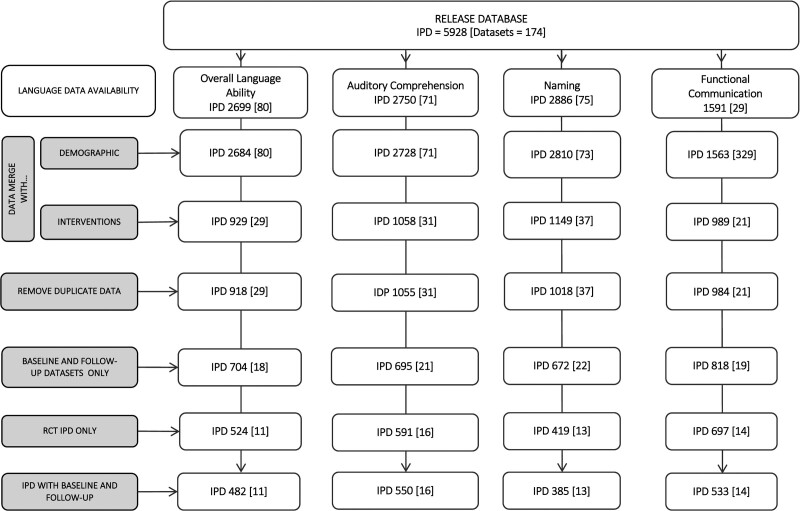
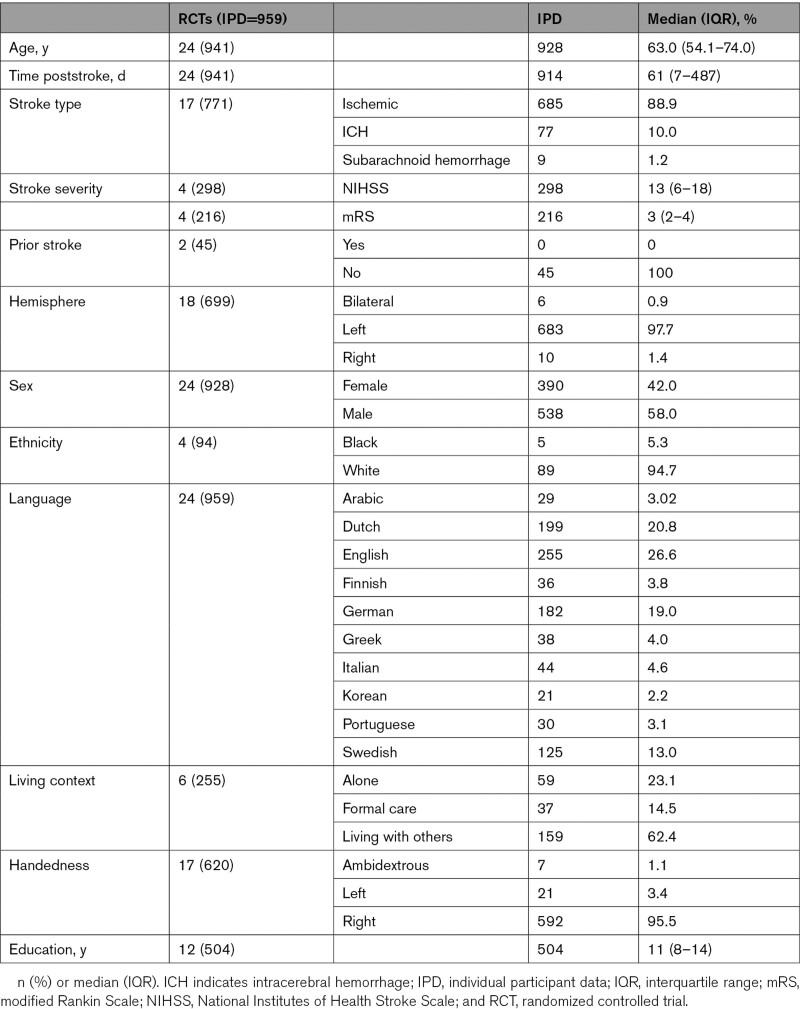
Duplicate IPD were removed and filtered by available demographic, language, and intervention data to support the analysis reported here (Figure 1). Network meta-analyses were based on overall language ability (482 IPD and 11 RCTs); functional communication (observer rated; 533 IPD and 14 RCTs), auditory comprehension (550 IPD and 16 RCTs), and naming outcomes (385 IPD and 13 RCTs; Figure 1). Of 10 languages represented, English speakers were most prevalent (255 IPD; 26.6%). Median time since stroke was 61 days (interquartile range, 7–487; 914 IPD) with left hemisphere (683 IPD; 97.7%) ischemic strokes (685 IPD; 88.9%) predominating (Table; Tables I through III in the Supplemental Material). Models were produced without within-study clustering effect. We examined within-study clustering, but findings were nonsignificant or caused a model failure as the G matrix was not positive definite (Table IV in the Supplemental Material).
Figure 1.
Individual participant data (IPD; data sets) by availability for network meta-analysis, language, demographics, and intervention. RCT indicates randomized controlled trial.
Table.
Characteristics of Included Participants by RCT Data Reported and by IPD Availability
IPD Network Meta-Analysis
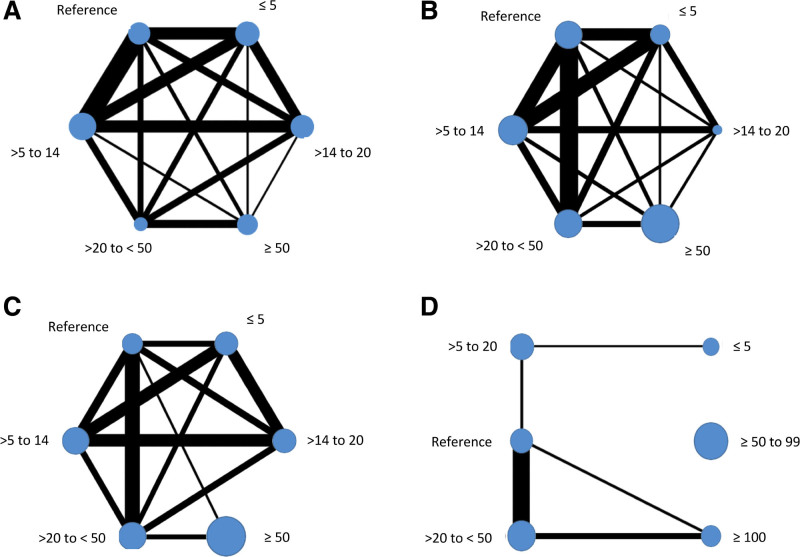
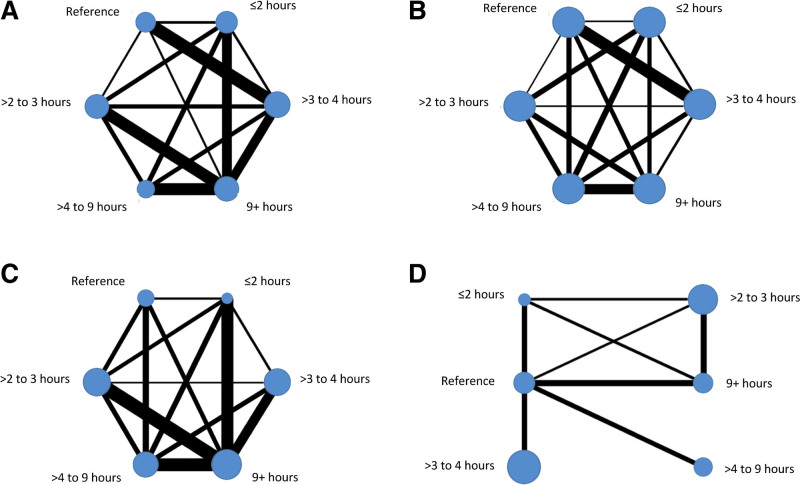
We mapped intervention comparisons that were direct (eg, an RCT comparison of intervention A versus B or B versus C) and indirect (comparisons that were not made within a specific RCT but could be made across RCTs based on the common intervention, in this example, A versus C). Networks were developed by language outcome. Interventions represented by a node were categorized by regimen (dosage [total SLT hours], intensity [SLT hours weekly], and frequency [days per week]), the language rehabilitation target and approach, SLT home practice, tailoring, context, provider, and delivery. Most language and intervention networks were stable (Figures 2 and 3; Figures II and IV in the Supplemental Material). The naming and duration networks were the exception. With limited nodal connections, caution should be used in interpretation.
Figure 2.
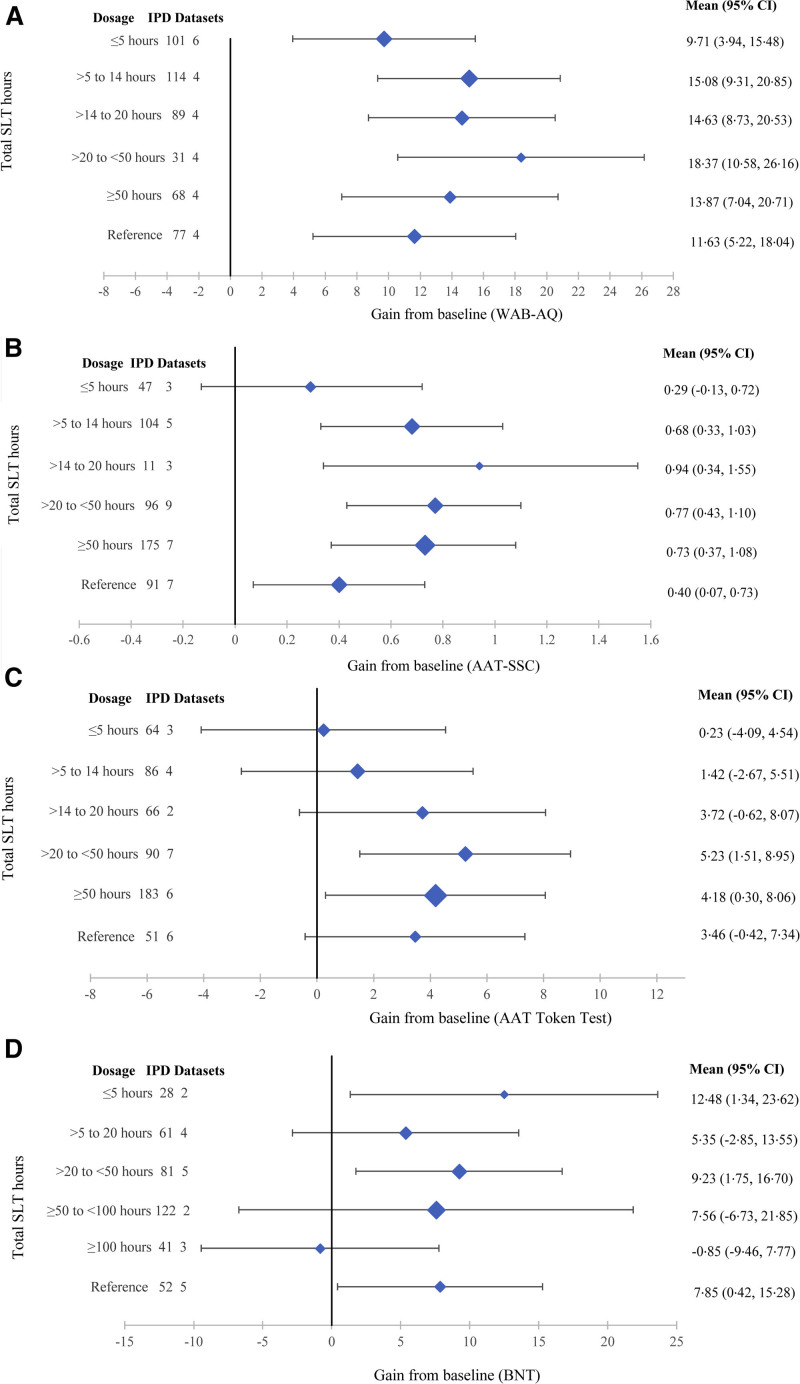
Dosage (total speech and language therapy hours) by language outcome. Overall language ability (A), functional communication (B), auditory comprehension (C), and naming (D).
Figure 3.
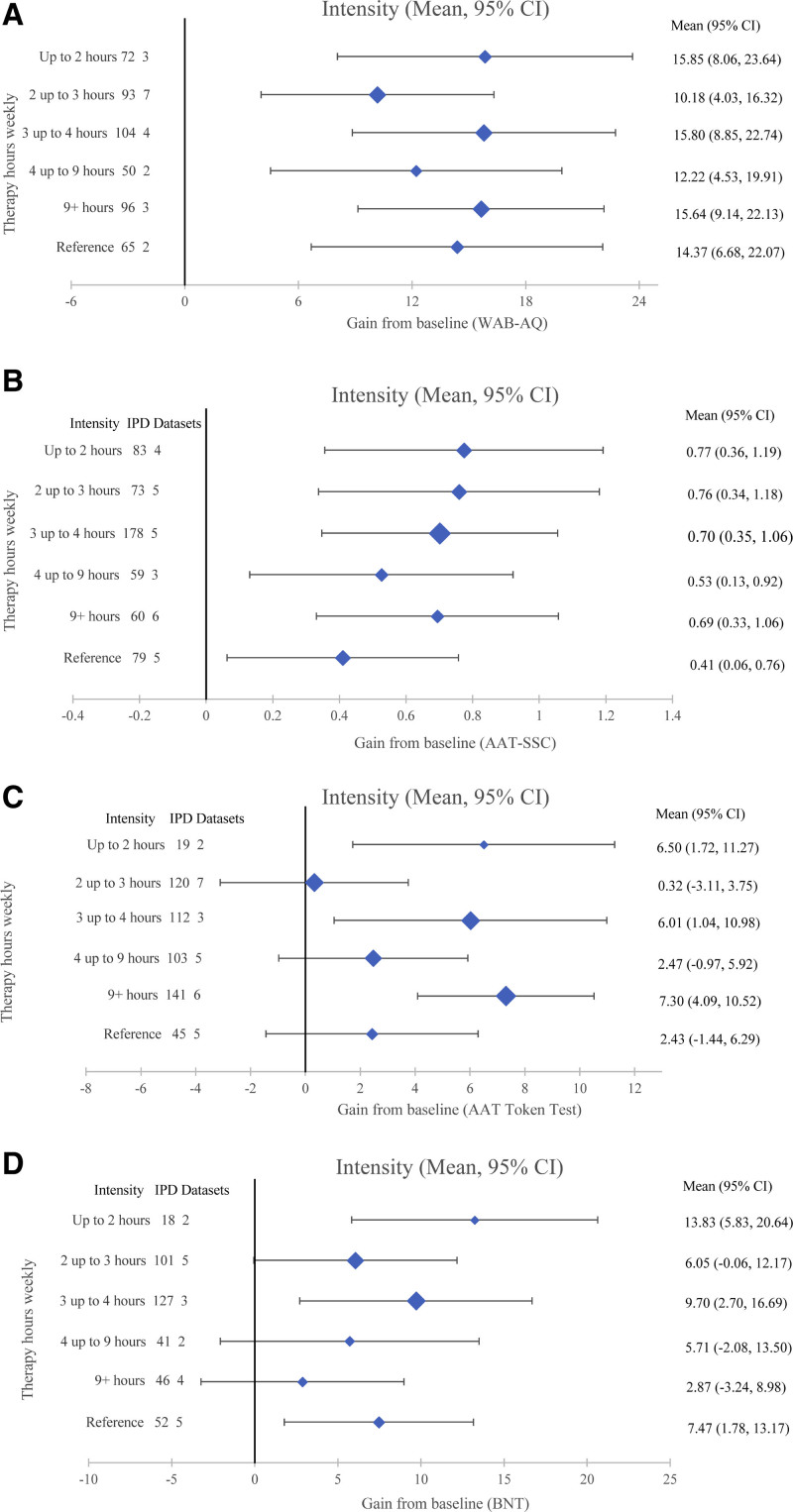
Intensity (speech and language therapy hours weekly) by language outcome. Overall language ability (A), functional communication (B), auditory comprehension (C), and naming (D).
SLT Dosage (Total SLT Hours)
Overall language (18.37 [10.58–26.16] WAB-AQ) and auditory comprehension gains (5.23 [1.51–8.95] AAT-TT) were the highest for 20 to 50 SLT hours within network meta-analyses involving 480 (11 RCTs) and 540 IPD (16 RCTs), respectively (Figure 4). Functional communication improvements (0.94 [0.34–1.55] AAT-SSC) were the greatest for 14 to 20 SLT hours but based on 11/524 IPD from 3/14 trials in the network. The next greatest gains occurred for 20 to 50 hours (0.77 [0.43–1.1]; 96 IPD and 9 RCTs) and 50+ hours (0.73 [0.37–1.08]; 175 IPD and 7 RCTs). No functional communication gains were observed for ≤5 hours SLT or comprehension gains for ≤20 hours SLT (Figure 4).
Figure 4.
Dosage (total speech and language therapy [SLT] hours) and associated gains from baseline (mean; 95% CI). Overall language (A): Western Aphasia Battery–Aphasia Quotient (0–100); 480 individual participant data (IPD; 11 randomized controlled trials [RCTs]); functional communication (B): Aachen Aphasia Test–Spontaneous Speech Communication (AAT-SSC; 0–5); 524 IPD (14 RCTs); auditory comprehension (C): Aachen Aphasia Test (AAT) Token Test (0–50); 540 IPD (16 RCTs); naming (D): Boston Naming Test (BNT; 0–60); 385 IPD (13 RCTs).
SLT Intensity (Hours Weekly)
Gains from baseline were observed across different SLT intensities for overall language (482 IPD and 11 RCTs) and functional communication (533 IPD and 14 RCTs). The greatest overall language gains were associated with ≤2 hours/week (15.85 [8.06–23.64] WAB-AQ; Figure 5A) with clinically equivalent gains 3 to 4 hours/week and 9+ hours/week (15.80 [8.85–22.74] and 15.64 [9.14–22.13]), respectively. Functional communication gains were the greatest for ≤2 hours/week (0.77 [0.36–1.19] AAT-SSC) with clinically equivalent gains for 2 to 3 hours/week (0.76 [0.34–1.18]) and 3-4 hours/week (0.70 [0.35–1.06]; Figure 5B). Auditory comprehension gains (540 IPD and 16 RCTs; Figure 5C) were numerically greatest for 9+ hours/week (7.3 [4.09–10.52] AAT-TT) with clinically similar gains for >3 to 4 hours/week (6.01 [1.04–10.98]) and up to 2 hours/week (6.5 [1.72–11.27]). Comprehension gains were not observed when SLT was 2 to 3 hours/week or 4 to 9 hours weekly (Figure 5C).
Figure 5.
Intensity (speech and language therapy hours/week) and associated gains from baseline (mean; 95% CI). Overall language (A): Western Aphasia Battery–Aphasia Quotient (0–100); 482 individual participant data (IPD; 11 randomized controlled trials [RCTs]); functional communication (B): Aachen Aphasia Test–Spontaneous Speech Communication (AAT-SSC; 0–5); 533 IPD (14 RCTs); auditory comprehension (C): Aachen Aphasia Test (AAT) Token Test (0–50); 540 IPD (16 RCTs); naming (D): Boston Naming Test (BNT; 0–60); 385 IPD (13 RCTs).
SLT Frequency (Days Weekly)
Overall language ability gains from baseline were evident across nodes (482 IPD and 11 RCTs). Numerically, the greatest gain was associated with 5 days/week (14.95 [8.67–21.23] WAB-AQ). Clinically similar gains were observed for 3 to 5+ days/week (Figure IIIA in the Supplemental Material) though 3 and 5+ days/week were based on fewer IPD and RCTs. For functional communication (526 IPD and 14 RCTs), gains were observed for SLT ≤5 days/week with the greatest numerical gain observed for 5 SLT days/week (0.78 [0.48–1.09] AAT-SSC; 155 IPD and 8 RCTs). Gains were not observed for SLT 5+ days/week. Auditory comprehension gains based on 540 IPD (16 RCTs) were only observed for 4 to 5 days/week with the numerically greatest associated with 4 days/week (5.86 [1.64–10.08]; Figures II and III in the Supplemental Material).
SLT Rehabilitation Target and Theoretical Approach
Few trials, IPD, and network connections informed the SLT target and theoretical approach analysis; thus cautious interpretation is warranted. The greatest overall language (15.62 [8.82–22.43] WAB-AQ) and functional communication gains (1.05 [0.52–1.58] AAT-SSC) occurred alongside mixed expressive-receptive targeted approaches. Auditory comprehension (4.46 [0.31–8.62] AAT-TT) and naming gains (8.82 [3.15–14.49] Boston Naming Test) were the greatest for word-finding approaches. Therapy targeting semantic-phonological recovery was associated with greater overall language ability (20.39 [1.90–38.88] WAB-AQ) and auditory comprehension gains from baseline (11.93 [1.44–22.43] AAT-TT) while functional/pragmatic approaches were associated with the greatest functional communication gains (1.82 [0.36–3.28] AAT-SSC; Tables V and VI in the Supplemental Material).
SLT Home Practice
Prescribed home practice regimen data were unavailable. Therefore, our analysis was based on whether home practice was an intervention component or not. Prescribed home practice was associated with greater gains in overall language (16.69 [10.01–23.37] WAB-AQ) and auditory comprehension (5.28 [2.19–8.37] AAT-TT). Where home practice was absent or unreported, functional communication gains were marginally higher (0.13+ points AAT-SSC) while naming gains were clinically equivalent (Table VII in the Supplemental Material).
SLT Tailoring, Context, Provider, and Delivery
No RCT directly compared tailored-to-untailored interventions. Where SLT was functionally tailored, language gains were greater for overall language ability (16.47 [10.95–21.99] WAB-AQ), naming (8.79 [1.95–15.63] Boston Naming Test), and marginally higher for functional communication (0.74 [0.38–1.10] AAT-SSC) than gains with untailored interventions. Auditory comprehension gains were only observed for functionally tailored therapy (5.26 points [2.05–8.47] AAT-TT).
Therapy tailored by difficulty was associated with auditory comprehension (4.57 [1.55–7.60]) and numerically greater overall language gains (14.4 [8.82–20.09] WAB-AQ) than untailored approaches (Table VIII in the Supplemental Material). Gains made from baseline were clinically equivalent across in- and outpatient settings, professionals or trained nonprofessional providers, and face-to-face, computer-supported, or self-managed therapy delivery approaches.
Risk of Bias
Risk of primary data set and meta-biases was moderate to low. All included interventions were confirmed as SLT and subcategorized through the REhabilitation and recovery of peopLE with Aphasia after StrokE collaborators’ consensus. Delivery and regimen differences were examined in a priori planned analyses. No analysis exceeded our prespecified threshold (50%) with relative variability 10% to 25%. Randomization was adequate (adequate random sequence generation, 17 RCTs [68%]; concealment of allocation, 15 [60%]), and 17 (68%) reported outcome assessor blinding. Attrition bias was low. Participants were retained and dropouts/nonadherence reported (Figure VI in the Supplemental Material). Where age, sex, time post-onset, and aphasia severity data allowed, participant groups were comparable at baseline. Sensitivity analyses found no evidence that historic data set exclusion, publication age, outcome measure choice, and fixed versus random effects models would have altered our findings (Tables IX through XII in the Supplemental Material).
Discussion
Our collaborative network synthesized 959 IPD (25 RCTs) in the largest stroke-related aphasia IPD network meta-analysis to date, reporting associations between therapy regimen and language gains. Controlling for age, sex, aphasia severity, and time poststroke at baseline, the greatest overall language and functional communication gains were associated with interventions that were mixed expressive-receptive approaches, delivered over 5 days weekly for up to 50 hours in total. Auditory comprehension gains were the greatest for word-finding SLT, for up to 9 hours weekly over 4 to 5 days for 20 to 50+ hours in total. Generally, language gains observed were the greatest when associated with interventions tailored by functional relevance and augmented by prescribed home practice tasks. Confirmation of the optimal dosage, intensities, and frequency will be achieved through definitive RCTs; however, current clinical provision falls below the therapy regimens associated in this study with the greatest language gains from baseline.10–15
Our novel IPD RCT network meta-analysis investigated associations between IPD and specific interventions across a range of language outcomes and offers insights into differential effects across prior RCTs. Intervention regimens associated with optimal recovery may vary by language outcome. Dosage, intensity, and frequency of interventions are important variables. Prescribed home practice and tailoring for relevance are essential considerations in future effectiveness RCTs of SLT. Previous SLTs for poststroke aphasia meta-analyses were limited to pairwise comparisons of aggregate data synthesis8 or small data sets and English-only publications.9
Strengths
We minimized the risk of selection and availability meta-biases and minimized data extraction and synthesis errors. Our study used a priori eligibility criteria, imposing no language, date, or publication limitations in our systematic data search, resulting in the inclusion of geographically and linguistically diverse data. We incorporated participant, study design, and IPD availability and SLT narrative descriptions in our analysis. Transformation of language data onto internationally recognized outcome measurement instruments ensured clinically meaningful change scores relevant to rehabilitation settings.
Limitations
Variations in demographic, language, and intervention data availability required the inclusion of many data sets to ensure sufficient overlap and support our preplanned analyses. Despite our extensive search followed by time-consuming IPD extraction and verification, some networks lacked randomized comparisons and our data set was predominantly from English-speaking participants, high-income countries, with well-developed stroke services.21 Given the augmented time and resource requirements for IPD meta-analyses (compared with aggregated metasynthesis approaches)53 and our last search date, our search strategy included trial registers. Potentially eligible ongoing trials were identified and invited to contribute their data once available, and consequently, our analysis based on 25 RCTs included data from 3 RCTs published in 2015 and 4 RCTs published 2016 to 2019.
Self-management, technology-facilitated, or therapist-trained nonprofessional SLT delivery models confer language benefits comparable to those achieved in traditional, face-to-face, one-to-one, therapist-led sessions8 and augment therapy dose within existing clinical resources. As a complex intervention, therapy frequency, intensity, and dosage are not entirely independent variables. Variations in intervention response among patient subgroups requires further investigation. Targeted trials to address network instabilities and confirm or refine our findings are required.
Implications
The dosage, intensity, and frequency of SLT regimens associated with the greatest overall language, functional communication, and auditory comprehension gains from baseline were higher than current clinical rehabilitation service reports.10–15 Therapy regimen, tailoring by functional relevance, and prescribed home practice are important considerations in establishing critical therapeutic ranges in clinical research contexts, which may vary by language outcome. These exploratory findings inform future hypothesis-testing trial designs and tailoring of clinical services.
Article Information
Acknowledgments
We thank the Collaboration of Aphasia Trialists (IS1208) EU Cooperation in Science and Technology and Patient and Public Involvement group members of the University of East Anglia Aphasia Research Collaboration.
Sources of Funding
This study was supported by the National Institute for Health Research Health Services and Delivery Research (14/04/22); The Tavistock Trust for Aphasia, United Kingdom. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the National Institute for Health Research, National Health Service, UK or the Department of Health, UK. All members of the REhabilitation and recovery of peopLE with Aphasia after StrokE collaboration had the opportunity to review and critically appraise the final draft of the manuscript.
Disclosures
During the conduct of the study, the authors report the following grants and awards: M.C. Brady: Chief Scientist Office, UK, European Union Cooperation in Science and Technology (IS1208), Glasgow Caledonian University Studentships, The Stroke Association, UK, Speech Pathology Australia Travel Grant, The Tavistock Trust for Aphasia, UK (TTA), and National Institute for Health Research, UK (NIHR), UK; A. Bowen and S.A. Thomas: NIHR and Stroke Association. C. Brandenburg: German Federal Ministry of Education and Research; E. Godecke: Western Australian State Health Research Advisory Council RSD-02720; 2008/9. N. Hawkins: NIHR; K. Hilari: Stroke Association, European Social Fund and Greek National Strategic Reference Framework and TTA. P. Jaecks: Weidmüller Stiftung. A.P.-H. Kong: National Institutes of Health (NIH). B. MacWhinney: NIH. R.S. Marshall: National Institute of Deafness and Other Communication Disorders and NIH; R. Palmer: NIHR and TTA. I. Papathanasiou: European Social Fund and Greek National Strategic Reference Framework. M. Rose: National Health and Medical Research Council (NHMRC), Australia. J.P. Szaflarski: NIH R01DC017137, R01 HD068488, R01 NS048281, and R15DC017280-01. I. van der Meulen: Stichting Rotterdams Kinderrevalidatiefonds Adriaanstichting, Stichting Afasie Nederland, Stichting Coolsingel and Bohn Stafleu van Loghum. L. Worrall: NHMRC. The other authors report no conflicts.
Supplemental Material
Tables I–XX
Figures I–VI
PRISMA Checklist
Supplementary Material
Appendix
The RELEASE Collaboration: Marian C. Brady (Glasgow Caledonian University, United Kingdom), Myzoon Ali (Glasgow Caledonian University, United Kingdom), Kathryn VandenBerg (Glasgow Caledonian University, United Kingdom), Linda J. Williams (University of Edinburgh, United Kingdom), Louise R. Williams (Glasgow Caledonian University, United Kingdom), Masahiro Abo (Jikei University School of Medicine, Japan), Frank Becker (University of Oslo, Sunnaas Rehabilitation Hospital, Norway), Audrey Bowen (MAHSC, University of Manchester, United Kingdom), Caitlin Brandenburg (The University of Queensland, Australia), Caterina Breitenstein (University of Muenster, Germany), Stefanie Bruehl (MAHSC, University of Manchester, United Kingdom; St. Mauritius Rehabilitation Centre and RWTH Aachen University, Germany), David A. Copland (The University of Queensland, Australia), Tamara B. Cranfill (Eastern Kentucky University), Marie di Pietro-Bachmann (University Hospital and University of Geneva, Switzerland), Pamela Enderby (University of Sheffield, United Kingdom), Joanne Fillingham (NHS Improvement, London, United Kingdom), Federica Lucia Galli (Marche Polytechnic University, Italy), Marialuisa Gandolfi (University of Verona, Italy), Bertrand Glize (University of Bordeaux, France), Erin Godecke (Edith Cowan University, Australia), Neil Hawkins (University of Glasgow, United Kingdom), Katerina Hilari (City, University of London, United Kingdom), Jacqueline Hinckley (Nova Southeastern University), Simon Horton (University of East Anglia, United Kingdom), David Howard (Newcastle University, Unitd Kingdom), Petra Jaecks (Bielefeld University, Germany), Elizabeth Jefferies (University of York, United Kingdom), Luis M.T. Jesus (University of Aveiro, Portugal), Maria Kambanaros (Cyprus University of Technology, Cyprus), Eun Kyoung Kang (Kangwon National University Hospital, Republic of Korea), Eman M. Khedr (Assiut University Hospital, Egypt), Anthony Pak-Hin Kong (University of Central Florida), Tarja Kukkonen (Tampere University Hospital, Finland), Marina Laganaro (University Hospital and University of Geneva, Switzerland), Matthew A. Lambon Ralph (University of Cambridge, United Kingdom), Ann Charlotte Laska (Karolinska Institutet, Sweden), Béatrice Leemann (Hôpitaux Universitaires de Genève, Switzerland), Alexander P. Leff (University College London, United Kingdom), Roxele R. Lima (Educational Association Bom Jesus–IELUSC, Brazil), Antje Lorenz (Humboldt University Berlin, Germany), Brian MacWhinney (Carnegie Mellon University), Rebecca Shisler Marshall (University of Georgia), Flavia Mattioli (Azienda Socio Sanitaria Territoriale, Italy), İlknur Maviş (Anadolu University, Turkey), Marcus Meinzer (University Medicine Greifswald, Germany), Reza Nilipour (University of Social Welfare and Rehabilitation Sciences, Iran), Enrique Noé (NEURORHB-Hospitales Vithas, Spain), Nam-Jong Paik (Seoul National University College of Medicine, Republic of Korea), Rebecca Palmer (University of Sheffield, United Kingdom), Ilias Papathanasiou (Technological Educational Institute of Western Greece, Greece), Brigida Patricio (School of Polytechnic Institute of Porto, Portugal), Isabel Pavão Martins (Universidade de Lisboa, Portugal), Cathy Price (University College London, United Kingdom), Tatjana Prizl Jakovac (University of Zagreb, Croatia), Elizabeth Rochon (University of Toronto, Canada), Miranda L. Rose (La Trobe University, Australia), Charlotte Rosso (Sorbonne Université and Hôpital Salpetriere, France), Ilona Rubi-Fessen (RehaNova Rehabilitation Hospital and University of Cologne, Germany), Marina B. Ruiter (Sint Maartenskliniek and Radboud University, Netherlands), Claerwen Snell (Warrington and Halton NHS Foundation Trust, United Kingdom), Benjamin Stahl (Charité Universitätsmedizin Berlin, Germany), Jerzy P. Szaflarski (University of Alabama at Birmingham), Shirley A. Thomas (University of Nottingham, United Kingdom), Mieke van de Sandt-Koenderman (University Medical Center Rotterdam, the Netherlands), Ineke van der Meulen (Erasmus University Medical Center, the Netherlands), Evy Visch-Brink (Erasmus University Medical Center, the Netherlands), Linda Worrall (The University of Queensland, Australia), Heather Harris Wright (North Carolina University).
Nonstandard Abbreviations and Acronyms
- AAT-SSC
- Aachen Aphasia Test–Spontaneous Speech Communication
- AAT-TT
- Aachen Aphasia Test–Token Test
- IPD
- individual participant data
- RCT
- randomized controlled trial
- SLT
- speech and language therapy
- WAB-AQ
- Western Aphasia Battery–Aphasia Quotient
A list of The REhabilitation and recovery of peopLE with Aphasia after StrokE (RELEASE) Collaborators is provided in the Appendix.
Supplemental Material is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.121.035216.
For Sources of Funding and Disclosures, see page 965.
References
- 1.The Global Burden of Diseases (GBD) 2016 Stroke Collaborators. Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:439–458. doi: 10.1016/S1474-4422(19)30034-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dickey L, Kagan A, Lindsay MP, Fang J, Rowland A, Black S. Incidence and profile of inpatient stroke-induced aphasia in Ontario, Canada. Arch Phys Med Rehabil. 2010;91:196–202. doi: 10.1016/j.apmr.2009.09.020 [DOI] [PubMed] [Google Scholar]
- 3.Bersano A, Burgio F, Gattinoni M, Candelise L; PROSIT Study Group. Aphasia burden to hospitalised acute stroke patients: need for an early rehabilitation programme. Int J Stroke. 2009;4:443–447. doi: 10.1111/j.1747-4949.2009.00349.x [DOI] [PubMed] [Google Scholar]
- 4.Paolucci S, Matano A, Bragoni M, Coiro P, De Angelis D, Fusco FR, Morelli D, Pratesi L, Venturiero V, Bureca I. Rehabilitation of left brain-damaged ischemic stroke patients: the role of comprehension language deficits. A matched comparison. Cerebrovasc Dis. 2005;20:400–406. doi: 10.1159/000088671 [DOI] [PubMed] [Google Scholar]
- 5.Hilari K, Northcott S. ‘Struggling to stay connected’: comparing the social relationships of healthy older people and people with stroke and aphasia. Aphasiology. 2016;31:674–687. [Google Scholar]
- 6.Gialanella B, Prometti P. Rehabilitation length of stay in patients suffering from aphasia after stroke. Top Stroke Rehabil. 2009;16:437–444. doi: 10.1310/tsr1606-437 [DOI] [PubMed] [Google Scholar]
- 7.Black-Schaffer RM, Osberg JS. Return to work after stroke: development of a predictive model. Arch Phys Med Rehabil. 1990;71:285–290. [PubMed] [Google Scholar]
- 8.Brady MC, Kelly H, Godwin J, Enderby P, Campbell P. Speech and language therapy for aphasia following stroke. Cochrane Database Syst Rev. 2016;2016:CD000425. doi: 10.1002/14651858.CD000425.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhogal SK, Teasell R, Speechley M. Intensity of aphasia therapy, impact on recovery. Stroke. 2003;34:987–993. doi: 10.1161/01.STR.0000062343.64383.D0 [DOI] [PubMed] [Google Scholar]
- 9a.Tierney JF, Vale C, Riley R, Tudur Smith C, Stewart L, Clarke M, Rovers M. Individual participant data (IPD) meta-analyses of randomised controlled trials: guidance on their use. PLOS Medicine. 2015;12:e1001855. doi: 10.1371/journal.pmed.1001855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palmer R, Dimairo M, Cooper C, Enderby P, Brady M, Bowen A, Latimer N, Julious S, Cross E, Alshreef A, et al. Self-managed, computerised speech and language therapy for patients with chronic aphasia post-stroke compared with usual care or attention control (Big CACTUS): a multicentre, single-blinded, randomised controlled trial. Lancet Neurol. 2019;18:821–833. doi: 10.1016/S1474-4422(19)30192-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Deleted in proof. [Google Scholar]
- 12.Kong AP-H, Tse CWK. Clinician survey on speech pathology services for people with Aphasia in Hong Kong. Clin Arch Commun Disord. 2018;3:201–212. [Google Scholar]
- 13.Rose M, Ferguson A, Power E, Togher L, Worrall L. Aphasia rehabilitation in Australia: current practices, challenges and future directions. Int J Speech Lang Pathol. 2014;16:169–180. doi: 10.3109/17549507.2013.794474 [DOI] [PubMed] [Google Scholar]
- 14.Guo YE, Togher L, Power E. Speech pathology services for people with aphasia: what is the current practice in Singapore? Disabil Rehabil. 2014;36:691–704. doi: 10.3109/09638288.2013.804597 [DOI] [PubMed] [Google Scholar]
- 15.Korsukewitz C, Rocker R, Baumgaertner A, Floel A, Grewe T, Ziegler W, Martus P, Schupp W, Lindow B, Breitenstein C. Wieder richtig sprechen lernen [evidence-based language rehabilitation]. Aerztliche Praxis Neurologie/Psychiatrie. 2013;4:24–26. [Google Scholar]
- 16.Pedersen PM, Vinter K, Olsen TS. Aphasia after stroke: type, severity and prognosis. The Copenhagen aphasia study. Cerebrovasc Dis. 2004;17:35–43. doi: 10.1159/000073896 [DOI] [PubMed] [Google Scholar]
- 17.Ali M, Lyden P, Brady M; VISTA Collaboration. Aphasia and Dysarthria in Acute Stroke: recovery and functional outcome. Int J Stroke. 2015;10:400–406. doi: 10.1111/ijs.12067 [DOI] [PubMed] [Google Scholar]
- 18.Brady MC, Ali M, VandenBerg K, Williams LJ, Williams LR, Abo M, Becker F, Bowen A, Brandenburg C, Breitenstein C, et al. RELEASE: a protocol for a systematic review based, individual participant data, meta- and network meta-analysis, of complex speech-language therapy interventions for stroke-related aphasia. Aphasiology. 2019;34:137–157. doi: 10.1080/02687038.2019.1643003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen JP, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–784. doi: 10.7326/M14-2385 [DOI] [PubMed] [Google Scholar]
- 20.Stewart LA, Clarke M, Rovers M, Riley RD, Simmonds M, Stewart G, Tierney JF; PRISMA-IPD Development Group. Preferred reporting items for systematic review and meta-analyses of individual participant data: the PRISMA-IPD statement. JAMA. 2015;313:1657–1665. doi: 10.1001/jama.2015.3656 [DOI] [PubMed] [Google Scholar]
- 21.The RELEASE Collaborators. Utilising a systematic review-based approach to create a database of individual participant data for meta- and network meta-analyses: the RELEASE database of aphasia after stroke. Aphasiology. 2021. doi: 10.1080/02687038.2021.1897081 [Google Scholar]
- 22.Greenland S, Senn SJ, Rothman KJ, Carlin JB, Poole C, Goodman SN, Altman DG. Statistical tests, P values, confidence intervals, and power: a guide to misinterpretations. Eur J Epidemiol. 2016;31:337–350. doi: 10.1007/s10654-016-0149-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Deleted in proof. [Google Scholar]
- 24.Breitenstein C, Grewe T, Flöel A, Ziegler W, Springer L, Martus P, Huber W, Willmes K, Ringelstein EB, Haeusler KG, et al. ; FCET2EC Study Group. Intensive speech and language therapy in patients with chronic aphasia after stroke: a randomised, open-label, blinded-endpoint, controlled trial in a health-care setting. Lancet. 2017;389:1528–1538. doi: 10.1016/S0140-6736(17)30067-3 [DOI] [PubMed] [Google Scholar]
- 25.The Rehabilitation and Recovery of People With Aphasia After Stroke (RELEASE) Collaborators. Predictors of post-stroke aphasia recovery: a systematic review-informed individual participant data (IPD) meta-analysis. Stroke. 2021;52:1778–1787. doi: 10.1161/STROKEAHA.120.031162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakamura A, Nakamura M. On the relationships among several specification error tests presented by Durbin, Wu, and Hausman. Econometrica. 1981;49:1583–1588. [Google Scholar]
- 27.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. (editors). Cochrane Handbook for Systematic Reviews of Interventions. 2019. 2nd Edition. Chichester (UK): John Wiley & Sons [Google Scholar]
- 28.Whiting P, Savović J, Higgins JP, Caldwell DM, Reeves BC, Shea B, Davies P, Kleijnen J, Churchill R; ROBIS Group. ROBIS: a new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol. 2016;69:225–234. doi: 10.1016/j.jclinepi.2015.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ciccone N, West D, Cream A, Cartwright J, Rai T, Granger A, Hankey GJ, Godecke E. Constraint-induced aphasia therapy (CIAT): a randomised controlled trial in very early stroke rehabilitation. Aphasiology. 2015;30:566–584. [Google Scholar]
- 30.Breitenstein C, Korsukewitz C, Baumgärtner A, Flöel A, Zwitserlood P, Dobel C, Knecht S. L-dopa does not add to the success of high-intensity language training in aphasia. Restor Neurol Neurosci. 2015;33:115–120. doi: 10.3233/RNN-140435 [DOI] [PubMed] [Google Scholar]
- 31.de Jong-Hagelstein M, van de Sandt-Koenderman WM, Prins ND, Dippel DW, Koudstaal PJ, Visch-Brink EG. Efficacy of early cognitive-linguistic treatment and communicative treatment in aphasia after stroke: a randomised controlled trial (RATS-2). J Neurol Neurosurg Psychiatry. 2011;82:399–404. doi: 10.1136/jnnp.2010.210559 [DOI] [PubMed] [Google Scholar]
- 32.Doesborgh SJC, van de Sandt-Koenderman MWME, Dippel DWJ, van Harskamp F, Koudstaal PJ, Visch-Brink EG. Cues on request: the efficacy of multicue, a computer program for wordfinding therapy. Aphasiology. 2004;18:213–222. [Google Scholar]
- 33.Doesborgh SJ, van de Sandt-Koenderman MW, Dippel DW, van Harskamp F, Koudstaal PJ, Visch-Brink EG. Effects of semantic treatment on verbal communication and linguistic processing in aphasia after stroke: a randomized controlled trial. Stroke. 2004;35:141–146. doi: 10.1161/01.STR.0000105460.52928.A6 [DOI] [PubMed] [Google Scholar]
- 34.Efstratiadou EA, Papathanasiou I, Holland R, Varlokosta S, Hilari K. Efficacy of elaborated semantic features analysis in aphasia: a quasi-randomised controlled trial. Aphasiology. 2019;33:1482–1503. doi: 10.1080/02687038.2019.1571558 [Google Scholar]
- 35.Godecke E, Hird K, Lalor EE, Rai T, Phillips MR. Very early poststroke aphasia therapy: a pilot randomized controlled efficacy trial. Int J Stroke. 2012;7:635–644. doi: 10.1111/j.1747-4949.2011.00631.x [DOI] [PubMed] [Google Scholar]
- 36.Khedr EM, Abo El-Fetoh N, Ali AM, El-Hammady DH, Khalifa H, Atta H, Karim AA. Dual-hemisphere repetitive transcranial magnetic stimulation for rehabilitation of poststroke aphasia: a randomized, double-blind clinical trial. Neurorehabil Neural Repair. 2014;28:740–750. doi: 10.1177/1545968314521009 [DOI] [PubMed] [Google Scholar]
- 37.Kukkonen T, Korpijaakko-Huuhka AM. How much is Enough and When is the Right Time? What Do We Know About the Good Practice and Timing of Aphasia Rehabilitation?. 2007. British Aphasiology Society [Google Scholar]
- 38.Laska AC, Kahan T, Hellblom A, Murray V, von Arbin M. A randomized controlled trial on very early speech and language therapy in acute stroke patients with aphasia. Cerebrovasc Dis Extra. 2011;1:66–74. doi: 10.1159/000329835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lincoln NB. An Investigation of the Effectiveness of Language Retraining Methods With Aphasic Stroke Patients. 1980. London: University of London. Ph.D. Thesis [Google Scholar]
- 40.Martins IP, Leal G, Fonseca I, Farrajota L, Aguiar M, Fonseca J, Lauterbach M, Gonçalves L, Cary MC, Ferreira JJ, et al. A randomized, rater-blinded, parallel trial of intensive speech therapy in sub-acute post-stroke aphasia: the SP-I-R-IT study. Int J Lang Commun Disord. 2013;48:421–431. doi: 10.1111/1460-6984.12018 [DOI] [PubMed] [Google Scholar]
- 41.Mattioli F, Ambrosi C, Mascaro L, Scarpazza C, Pasquali P, Frugoni M, Magoni M, Biagi L, Gasparotti R. Early aphasia rehabilitation is associated with functional reactivation of the left inferior frontal gyrus: a pilot study. Stroke. 2014;45:545–552. doi: 10.1161/STROKEAHA.113.003192 [DOI] [PubMed] [Google Scholar]
- 42.Meikle M, Wechsler E, Tupper A, Benenson M, Butler J, Mulhall D, Stern G. Comparative trial of volunteer and professional treatments of dysphasia after stroke. Br Med J. 1979;2:87–89. doi: 10.1136/bmj.2.6182.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meinzer M, Streiftau S, Rockstroh B. Intensive language training in the rehabilitation of chronic aphasia efficient training by laypersons. J Int Neuropsychol Soc. 2007;13:846–853. doi: 10.1017/S1355617707071111 [DOI] [PubMed] [Google Scholar]
- 44.Palmer R, Enderby P, Cooper C, Latimer N, Julious S, Paterson G, Dimairo M, Dixon S, Mortley J, Hilton R, et al. Computer therapy compared with usual care for people with long-standing aphasia poststroke: a pilot randomized controlled trial. Stroke. 2012;43:1904–1911. doi: 10.1161/STROKEAHA.112.650671 [DOI] [PubMed] [Google Scholar]
- 45.Rodriguez AD, Worrall L, Brown K, Grohn B, McKinnon E, Pearson C, Van Hees S, Roxbury T, Cornwell P, MacDonald A, et al. Aphasia LIFT: exploratory investigation of an intensive comprehensive aphasia programme. Aphasiology. 2013;27:1339–1361. [Google Scholar]
- 46.Rubi-Fessen I, Hartmann A, Huber W, Fimm B, Rommel T, Thiel A, Heiss WD. Add-on effects of repetitive transcranial magnetic stimulation on subacute Aphasia Therapy: enhanced improvement of functional communication and basic linguistic skills. A Randomized Controlled Study. Arch Phys Med Rehabil. 2015;96:1935–44.e2. doi: 10.1016/j.apmr.2015.06.017 [DOI] [PubMed] [Google Scholar]
- 47.Szaflarski JP, Ball AL, Vannest J, Dietz AR, Allendorfer JB, Martin AN, Hart K, Lindsell CJ. Constraint-induced aphasia therapy for treatment of chronic post-stroke aphasia: a randomized, blinded, controlled pilot trial. Med Sci Monit. 2015;21:2861–2869. doi: 10.12659/MSM.894291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smania N, Aglioti SM, Girardi F, Tinazzi M, Fiaschi A, Cosentino A, Corato E. Rehabilitation of limb apraxia improves daily life activities in patients with stroke. Neurology. 2006;67:2050–2052. doi: 10.1212/01.wnl.0000247279.63483.1f [DOI] [PubMed] [Google Scholar]
- 49.Smania N, Girardi F, Domenicali C, Lora E, Aglioti S. The rehabilitation of limb apraxia: a study in left-brain-damaged patients. Arch Phys Med Rehabil. 2000;81:379–388. doi: 10.1053/mr.2000.6921 [DOI] [PubMed] [Google Scholar]
- 50.Van Der Meulen I, Van De Sandt-Koenderman MW, Heijenbrok MH, Visch-Brink E, Ribbers GM. Melodic intonation therapy in chronic aphasia: evidence from a pilot randomized controlled trial. Front Hum Neurosci. 2016;10:533. doi: 10.3389/fnhum.2016.00533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Woodhead ZV, Crinion J, Teki S, Penny W, Price CJ, Leff AP. Auditory training changes temporal lobe connectivity in ‘Wernicke’s aphasia’: a randomised trial. J Neurol Neurosurg Psychiatry. 2017;88:586–594. doi: 10.1136/jnnp-2016-314621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.You DS, Kim DY, Chun MH, Jung SE, Park SJ. Cathodal transcranial direct current stimulation of the right Wernicke’s area improves comprehension in subacute stroke patients. Brain Lang. 2011;119:1–5. doi: 10.1016/j.bandl.2011.05.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.