Abstract
Background:
Addition of the BCL2 inhibitor venetoclax to lower intensity therapy improved survival in older and unfit patients with newly diagnosed acute myeloid leukemia (AML). The aim of this completed study was to investigate the activity of venetoclax combined with intensive chemotherapy.
Methods:
In this cohort of a larger phase 2 study we investigated the efficacy and safety of venetoclax plus CLIA in frontline patients with AML and MDS (NCT02115295). Patients ≤ 65 years of age, fit for intensive therapy, received cladribine (5 mg/m2), cytarabine (1.5 g/m2 for <60 and 1 g/m2 for ≥ 60) on days (D) 1-5 and idarubicin (10 mg/m2) on D1-3. Consolidation was cladribine (5 mg/m2) and cytarabine (1 g/m2 for <60 and 0.75 g/m2 for ≥ 60) on D1-3 and idarubicin (8 mg/m2) on D1-2. Venetoclax (400 mg) was given on D2-8 with each course. The primary objective was composite complete response (CR) rate. Secondary objectives were overall response (ORR), duration of response (DOR), event-free survival (EFS), overall survival (OS), and safety.
Findings:
50 patients were enrolled. Median age was 48 years (IQR, 37-56). CR+CRi was noted in 47 patients (94%); 37 of 45 (82%) had undetectable measurable residual disease (MRD); 3 patients did not respond, the ORR was 94%. The median DOR, EFS, and OS are not reached. The estimated 12-month DOR, EFS, and OS rate are 72% (95% CI: 58-90%), 68% (95% CI: 54-85%), and 85% (95% CI: 75-97%), respectively. The most common Grade ≥ 3 adverse events were neutropenic fever (n=42), infection (n=6), and ALT elevations (n=6). There was one induction death in a patient treated with CLIA-venetoclax plus a FLT3 inhibitor.
Interpretation:
Venetoclax added to CLIA was safe and effective in newly diagnosed AML and high risk MDS, producing high rates of durable MRD negative remissions and encouraging EFS and OS across prognostic subgroups.
Funding:
This trial was supported by M.D. Anderson Cancer Center Leukemia SPORE P50CA100632 and Cancer Center Support Grant P30CA016672.
INTRODUCTION
For several decades, intensive chemotherapy for remission induction in newly diagnosed AML consisted of a combination of cytarabine and an anthracycline. Over the years modifications to the standard backbone have been implemented, resulting in higher response rates, deeper remissions, and steadily improving survival in younger and fit patients. The incorporation of high-dose cytarabine into induction therapy has been shown to improve response rates and OS in younger patients with newly diagnosed AML(1-3). Similarly, optimization of the anthracycline dose-schedules demonstrated benefit(4). Lowenberg et al randomized 813 older patients and Fernandez et al randomized 657 younger patients with newly diagnosed AML to daunorubicin 45 mg/m2 or 90 mg/m2 as part of a 7+3 regimen(5, 6), observing higher rates of CR and superior OS with higher dose daunorubicin, with the greatest benefit seen in younger patients. Subsequent studies have determined that an intermediate daunorubicin dose of 60 mg/m2 achieved the optimal balance of safety and efficacy(7, 8). Alternative anthracyclines such as idarubicin were equivalent or superior to daunorubicin. In a randomized trial by the EORTC-GIMEMA, investigators observed improved rates of 5-year disease-free and OS with idarubicin compared to daunorubicin(9). In a randomized study by Pautas et al, 3 days of idarubicin 12 mg/m2 resulted in higher rates of CR and 4-year EFS compared with daunorubicin 80 mg/m2 for 3 days(10).
Adenosine nucleoside analogues have been added to the cytarabine and anthracycline backbone to try and further improve outcomes. Purine nucleoside analogues such as fludarabine, clofarabine, or cladribine have been shown to increase intracellular uptake and retention of cytarabine, leading to heightened leukemic cell killing(11). Regimens such as FLAG (fludarabine, cytarabine, GCSF) or FLAG-Idarubicin have successfully translated into the clinic. More recently, a series of studies by the Polish Adult Leukemia Group (PALG) have demonstrated the improved efficacy of cladribine added to 7+3 in patients with newly diagnosed AML(12). In a subsequent randomized trial, Holowiecki, et al demonstrated that cladribine, but not fludarabine improved outcomes when added to 7+3 in newly diagnosed AML(13).
Based on these prior studies, we designed a regimen that incorporated the stepwise modifications to optimize frontline therapy for younger and fit patients with newly diagnosed AML. The CLIA regimen combines cladribine with higher doses of cytarabine (1-1.5 grams/m2) and idarubicin. Among 47 patients with newly diagnosed AML with a median age of 54 years, we reported a CR/CRp (CR with incomplete platelet recovery) rate of 81% (55% were MRD negative rate at response), a 4-week mortality rate of 0%, and median OS not reached. The estimated 1-year OS rate was 75%(14).
Recently, the BCL-2 inhibitor venetoclax has demonstrated significant activity in combination with hypomethylating agents in older and unfit patients with newly diagnosed AML(15-17). In the VIALE-A study, 431 patients (median age of 76 years) were randomized to 5-azacitidine with or without venetoclax. The combination was associated with significant improvements in rates of CR/CRi (36.7% vs. 17.9%; P<0.001) and median OS (14.7 vs. 9.6 months; P<0.01) compared with 5-azacitidine alone. This combination is now the new standard of care in newly diagnosed older/unfit AML.
To further optimize frontline therapy for younger and fit patients, and based on evidence of its activity in combination with lower intensity therapy, we investigated the safety and efficacy of venetoclax combined with CLIA in newly diagnosed younger/fit AML.
METHODS
Study Design and Participants:
Patients 65 years of age or younger with a diagnosis of AML, mixed phenotype acute leukemia (MPAL), or high-risk myelodysplastic syndrome (MDS) (>/= 10% blasts or IPSS >/= intermediate-2) were eligible. Patients were eligible if they had newly diagnosed AML/MDS with no prior potentially curative therapy for leukemia. Prior therapy with hydroxyurea, hematopoietic growth factors, azacitidine, decitabine, ATRA, or a total dose of cytarabine up to 2g (for emergent cytoreduction) was allowed. Other eligibility criteria required a good performance status and organ functions (detailed in the protocol under cohort 4). A full list of the inclusion and exclusion criteria are included in the supplemental appendix (appendix p9). All investigations were conducted under approval of the institutional review committee and in accordance with the declaration of Helsinki.
Cytogenetic evaluation used standard metaphase karyotype analysis. Molecular analyses via an 81-gene institutional next-generation sequencing platform were performed at study enrollment(18). Measurable residual disease (MRD) was assessed by 8-color multiparameter flow cytometry using a combined assessment of “different from normal” and leukemia associated immunophenotype with a minimum sensitivity of 10−3-10−4 (0.1-0.01%)(19).
The CLIA trial protocol was amended to add an independent cohort ‘4’ to investigate the safety and activity of venetoclax added to intensive chemotherapy. To establish tolerability and safety of the venetoclax dose, a 3+3 dose-escalation/de-escalation safety lead-in was used to confirm safety. The first 3 evaluable patients were treated at the −1 dose level of venetoclax (200 mg of venetoclax on days 2-8 with adjustments for CYP3A inhibitors). If this was deemed safe, the next 3 evaluable patients were treated at dose level 1 (400 mg). Dose level 1 was the recommended phase II dose (RP2D) and continued for additional patients enrolled (detailed in the protocol under cohort 4).
Procedures:
CLIA induction consisted of 28-day courses of intravenous (IV) cladribine (5 mg/m2 IV over 1-2 hours and cytarabine (1.5 g/m2 for those <60 years old and 1 g/m2 for those ≥ 60 years old) IV over 2 hours on days (D) 1-5 with cytarabine infusion starting 3-6 hours following the start of the cladribine infusion. Idarubicin was given at a dose of 10 mg/m2 IV on D1-3. Consolidation consisted of 28-day courses of IV cladribine (5 mg/m2) and cytarabine (1 g/m2 for those <60 years old and 0.75 g/m2 for those ≥ 60 years old) on D1-3 and idarubicin 8 mg/m2 IV on D1-2 (appendix p1). Venetoclax was given for seven days per course on D2-8 without ramp up, however cytoreduction was utilized prior to therapy for patients with WBC > 20 k/μl (detailed in the protocol under cohort 4). Giving the venetoclax starting on day 2 allowed at least one day of intensive chemotherapy for cytoreduction in order to mitigate the risk of TLS that could occur with higher peripheral blasts and burden of disease. Venetoclax starting dose was 400 mg daily and dose adjustments were implemented for patients receiving CYP3A inhibitors such as azole antifungals. Patients receiving strong or moderate CYP3A inhibitors received 100 mg or 200 mg of venetoclax, respectively (detailed in the protocol under cohort 4). Patients were hospitalized during the initial course of therapy and then followed at least weekly for blood counts and chemistry labs. Antimicrobial prophylaxis with antibacterial, antiviral, and antifungal was applied during periods of neutropenia. In the absence of treatment delays due to adverse events, the treatment could be continued for up to 6 courses. Eligible patients were offered allogeneic stem cell transplant (alloSCT) in first CR. Otherwise, the patients continued on the study unless they developed progressive disease, unacceptable toxicity, or stopped for physician/patient preference.
Patients with a known FLT3-ITD or –TKD mutation could receive midostaurin at 50mg orally twice daily on days 6-19 during induction, and then on days 6-19 during consolidation; or gilteritinib at 120 mg daily on D1-14 of the induction course and then continuously during consolidation courses.
Outcomes:
The primary objective was to determine the composite complete response (CR) rate of venetoclax plus CLIA. Composite CR rate was defined as patients achieving a CR or CR with incomplete blood count recovery (CRi) per modified IWG criteria(20). Secondary objectives included assessing the overall response rate (ORR) (CR+CRi+PR), OS, EFS, duration of response (DOR), as well as the safety and tolerability of this regimen. Events in EFS included relapse, death, and non-response to therapy. Adverse events were assessed according to NCI CTCAE 4.0. All patients who received any treatment on protocol were included in the analysis.
Statistical Analysis:
A sample size of up to 50 patients was planned to achieve a posterior 95% credible interval width of less than 30% assuming a composite CR rate of 0.45 with a prior of beta (0.9, 1.1). A 95% credible interval, which is the central portion of the estimated posterior distribution that contains 95% of the values will be calculated for the primary objective. Futility and toxicity monitoring employed a Bayesian method(21) (detailed in the protocol under cohort 4). 95% credible intervals was calculated for the primary objective with a prior for composite complete response of beta (0.9, 1.1) using Parameter Solver 3.0 (https://biostatistics.mdanderson.org/SoftwareDownload/SingleSoftware/Index/6). 95% exact Blyth-Still-Casella confidence intervals (95% CI) were computed for other response outcomes using StatXact-11 (Cytel Inc.). Descriptive statistics were presented in tables and figures. Time-to-event analyses were estimated using the Kaplan-Meier method and differences were compared using log-rank tests and Cox proportional hazards models for the primary study populations and exploratory subgroup analyses. All statistical analyses were performed in R (version 4.0.2) unless otherwise specified. This trial was registered at clinicaltrials.gov as NCT02115295.
Role of the Funding Source:
The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
RESULTS
The baseline patient characteristics are summarized in Table 1. Between February 25, 2019 and March 23, 2021, 50 patients were enrolled, including 45 (90%) patients with AML, 4 (8%) with MDS, and 1 (2%) with MPAL (Figure 1). The median age was 48 years [range, 18 – 64 years; interquartile range (IQR) 37 to 56 years] and 28 (56%) were men. Among patients with AML, 16 (35%) were risk-stratified as favorable, 14 (30%) as intermediate, and 16 (35%) as adverse risk per European Leukemia Network (ELN) prognostic group. Two AML patients had isolated myeloid sarcoma without bone marrow involvement. Among the patients with MDS, 2 (50%) were high and two (50%) were very-high risk based on Revised International Prognostic Scoring System (IPSS-R). The patient with MPAL had a T/myeloid phenotype with a predominant myeloid phenotype (Table 1).
Table 1:
Baseline Characteristics
| N = 50 | |
|---|---|
| Age | 48 (18 - 64) |
| Race | |
| White | 30 / 50 (60%) |
| Black | 10 / 50 (20%) |
| Asian | 2 / 50 (4.0%) |
| Hispanic | 1 / 50 (2.0%) |
| Other | 7 / 50 (14%) |
| Gender | |
| Female | 22 / 50 (44%) |
| Male | 28 / 50 (56%) |
| Diagnosis | |
| AML | 45 / 50 (90%) |
| MDS | 4 / 50 (8.0%) |
| MPAL | 1 / 50 (2.0%) |
| Cytogenetic Group | |
| Favorable | 1 / 50 (2.0%) |
| Diploid | 25 / 50 (50%) |
| Other Intermediate | 8 / 50 (16%) |
| Adverse/Complex | 10 / 50 (20%) |
| Insufficient Mitoses | 5 / 50 (10%) |
| ELN Risk | |
| Favorable | 16 / 46 (35%) |
| Intermediate | 14 / 46 (30%) |
| Adverse | 16 / 46 (35%) |
| IPSS-R | |
| High | 2 / 4 (50%) |
| Very High | 2 / 4 (50%) |
|
Baseline Bone
Marrow Blasts |
|
| AML | 59 (1 – 84) |
| MDS | 9.5 (8-18) |
| MPAL | 82 |
| Baseline WBC | 6 (1 - 70) |
| Baseline Peripheral Blasts | 20 (1 - 95) |
| Baseline Hemoglobin | 8.60 (7.00 - 13.20) |
| Baseline Platelet Count | 44 (6 - 398) |
| Baseline Total Bilirubin | 0.40 (0.20 - 1.30) |
| Baseline Serum Creatinine | 0.84 (0.50 - 1.39) |
Median (Range); n / N (%)
Figure 1. Trial profile.
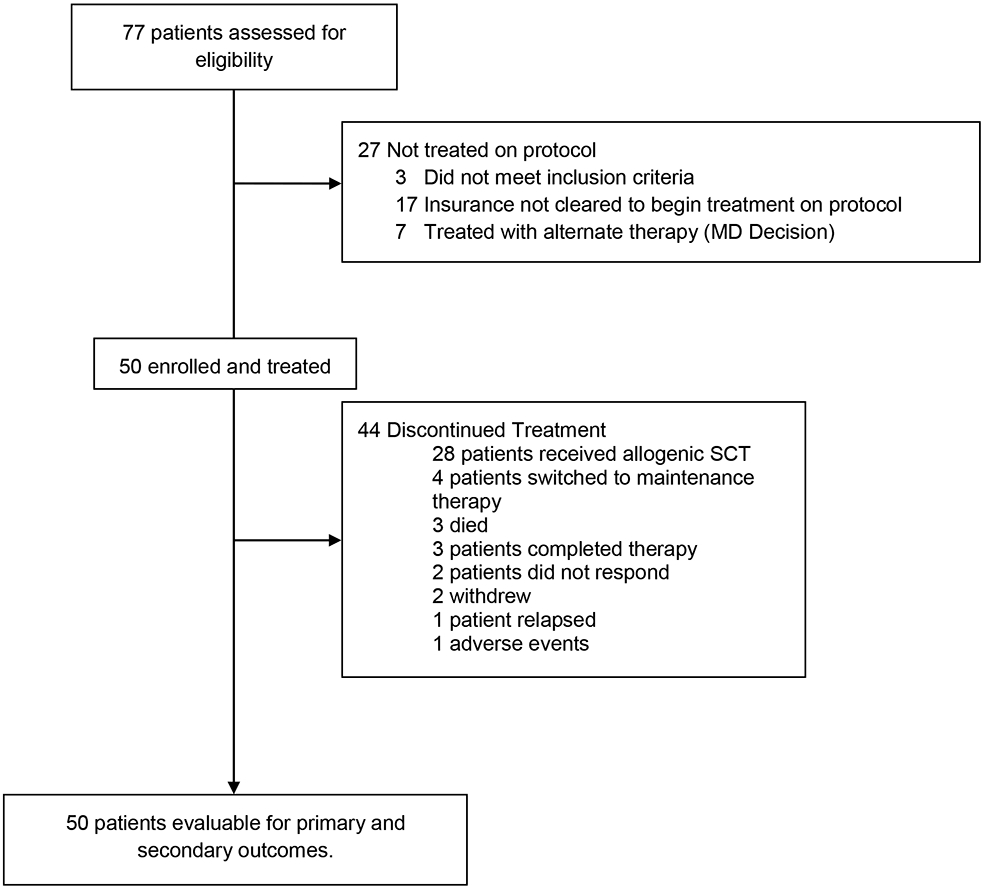
HSCT=haematopoietic stem-cell transplantation.
Favorable risk cytogenetics (core-binding factor) were present in one patient whose initial marrow was insufficient for cytogenetics, but peripheral blood FISH showed RUNX1-RUNXT1/t(8;21) after the patient had started treatment on protocol. Diploid karyotype was present in 50% (25/50). Other intermediate risk (non-diploid) karyotype was present in 16% (8/50), and adverse-risk/complex cytogenetics were present in 20% (10/50) of patients. Baseline cytogenetics were not available in 5 (10%) of patients because of insufficient metaphases obtained for analysis (Table 1). Mutations in DNMT3A (38%), NPM1 (36%), RAS (26%), FLT3-ITD (20%), FLT3-TKD (14%), RUNX1 (12%), TET2 (12%), ASXL1 (10%), IDH2 (10%), and IDH1 (8%), were common (appendix p2).
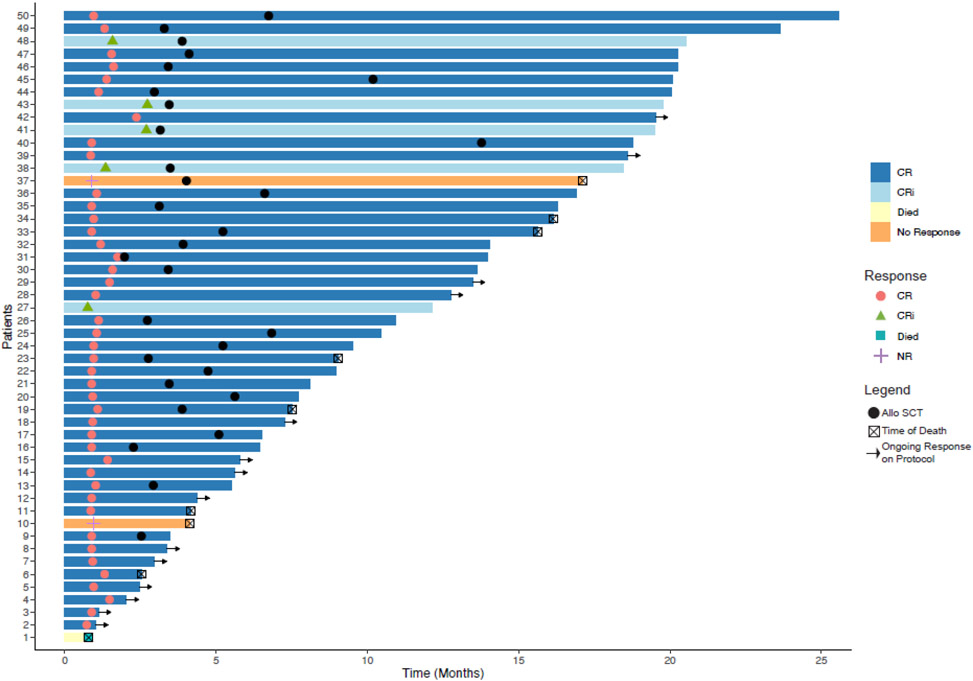
Patients with proliferative disease at presentation were allowed to receive limited therapy for emergent cytoreduction prior to enrollment. No patients with MDS required emergent cytoreduction. 29 patients (58%) required emergent cytoreduction, with 27 patients (54%) receving hydroxyruea at a median cumulative dose of 10 g (IQR: 6.75-15 g), 12 patients (24%) receving cytarabine at a median cumulative dose of 2 g (IQR: 1.375-2 g), and 3 patients (6%) reciving ATRA at a median dose of 60 mg (IQR: 50-105 mg). No patient achieved a CR/CRi with emergent cytoreduction alone. Patients received a median of two treatment courses (range 1 - 5, Table 2); 64% (N=32) received 1-2 courses; 36% (N=18) received ≥3 courses. The median time to count recovery in responding patients following induction (ANC ≥ 1000 and platelet count ≥ 50,000) was 27 days (IQR: 25 - 37 days). Count recovery data included only responding patients, with the exception of the one patient that died during induction. Non-responding patients had persistent disease hampering count recovery or received salvage therapy which would have interfered with our assessment of count recovery and thus were excluded from this analysis. The median time to recovery of absolute neutrophil count ≥ 1000/μL was 29 days (IQR: 26 - 37) and to platelet count ≥ 50k and 100k/μL were 24 days (IQR: 21 - 32) and 27 days (IQR: 23 - 34), respectively after induction. Course length extending ≥45 days occurred in only three (6%) patients (median age 57 years; IQR: 52-58) following induction therapy. At the data cutoff, 29 patients (62%) had proceeded to alloSCT in remission after a median of two (IQR: 2 - 3) courses. One non-responding patient underwent an alloSCT after response to salvage therapy in CR2 (Figure 2B). The 18 responding patients (36%) who have not undergone alloSCT have thus far received a median of 2.5 (range: 1 – 5; IQR: 2 - 3) courses of therapy; 50% (n=9) received ≥3 treatment courses (Table 2).
Table 2:
Response Assessment
| Overall, N = 50 |
CLIA+Venetoclax, N = 41 |
CLIA+Venetoclax+FLT3i, N = 9 | |
|---|---|---|---|
| Response Rate (CR+CRi) | 47 / 50 (94%) | 39 / 41 (95%) | 8 / 9 (89%) |
| Overall Response Rate (CR+CRi) | 47 / 50 (94%) | 39 / 41 (95%) | 8 / 9 (89%) |
| Best Response | |||
| CR | 42 / 50 (84%) | 35 / 41 (85%) | 7 / 9 (78%) |
| CRi | 5 / 50 (10%) | 4 / 41 (9.8%) | 1 / 9 (11%) |
| NR | 2 / 50 (4.0%) | 2 / 41 (4.9%) | 0 / 9 (0%) |
| Died | 1 / 50 (2.0%) | 0 / 41 (0%) | 1 / 9 (11%) |
| MRD Negative after Induction | 32 / 45 (71%) | 28 / 39 (72%) | 4 / 8 (50%) |
| MRD Negative on Study | 37 / 45 (82%) | 32 / 37 (94%) | 5 / 8 (62%) |
| Total Number of Courses Given | 2.00 (1.00 - 5.00) | 2.00 (1.00 - 5.00) | 2.00 (1.00 - 3.00) |
| Time to count recovery After induction (Days) | 27 (18 - 61) | 27 (18 - 61) | 38 (26 - 42) |
| Responders that Received alloSCT | 29 / 47 (62%) | 23 / 39 (60%) | 6 / 8 (75%) |
| Mortality Rate at One Month | 1 / 50 (2.0%) | 0 / 41 (0%) | 1 / 9 (11%) |
| Mortality Rate at Two Months | 1 / 48 (2.1%) | 0 / 39 (0%) | 1 / 9 (11%) |
n / N (%); Median (Range)
Figure 2. Swimmer plot of dynamic response assessment.
Each bar is an individual patient, colour coded by response. HSCT=haematopoietic stem-cell transplantation.
15 patients had a FLT3-ITD and/or -TKD mutation (8 with FLT3-ITD, 5 with FLT3-TKD, and 2 with both, appendix p2). 9 of these patients received a concomitant FLT3 inhibitor (FLT3i) during induction and consolidation. Compared to patients receiving venetoclax alone, the 9 receiving venetoclax plus CLIA and a FLT3i experienced delayed count recovery (median 38 vs 27 days, p = 0.01). 8 of the 9 patients treated with a FLT3i received gilteritinib with one patient receiving midostaurin.
A total of 47 patients achieved a CR + CRi (94%), including 42 CRs (84%) and 5 CRi (10%). The Bayesian posterior estimate for the probability of a CR or CRi and 95% credible interval are 0.92 (0.83, 0.98). The median number of courses given to response was 1 (range: 1-2). Of the 45 responding patients eligibile for MRD assessment, 37 (82%) attained MRD negativity while on the study (95% CI: 68%, 92%). 2 patients with isolated myeloid sarcoma without medullary disease at diagnosis were not evaluable for MRD assessment. The median DOR is not reached. The estimated DOR rate at 12 months was 72% (95% CI: 58-90%) (appendix p3). Two patients had no response to induction therapy and 1 patient died during induction prior to response assessment. Of the two patients who did not respond one had ELN adverse risk disease with complex karyotype and a TP53 mutation and the other patient had t(8;21), core binding factor AML. Among the nine patients treated with a concomitant FLT3i, eight (89%; 95% CI: 56%,99%) responded (7 CR + 1 CRi). Response dynamics are summarized in the swimmer plot (Figure 2).
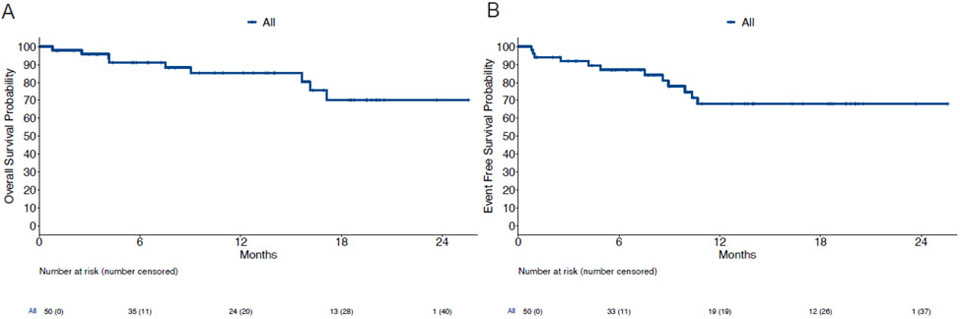
With a median follow-up of 13.5 months (IQR: 6.4-19.5 months), the median OS has not yet been reached; the estimated 12-month OS rate was 85% (95% CI: 75-97%) (Figure 3A). The median EFS has not been reached, with the estimated 12-month EFS rate was 68% (95% CI: 54-85%) (Figure 3B). Twenty-nine of the 47 responding patients (62%) underwent later alloSCT, alloSCT censored OS is noted in Figure S3 (appendix p4). Patients with ELN intermediate or adverse risk, or those with persistent MRD were referred for allogeneic stem cell transplant in first remission. Figure S4 shows overall survival of responding patients by receipt of allogeneic SCT and similar OS between groups (appendix p5). The efficacy of the regimen was noted across risk groups with estimated 12-month survival rates of 78% (95% CI: 59-100%), 93% (95% CI: 80-100%), and 81% (95% CI: 60-100%) among favorable, intermediate, and adverse risk ELN risk groups, respectively (appendix p6). Patients receiving a concomitant FLT3i had a similar OS (p=0.38) and EFS (p=0.20) compared with those receiving venetoclax plus CLIA alone (appendix p7). Among the responders, the achievement of an MRD negative state did not appear to affect OS (p=0.33) or EFS (p=0.67) (appendix p8).
Figure 3. Kaplan-Meier plots of overall survival and event-free survival.
(A) Overall survival of all patients. (B) Event free survival of all patients
Adverse events (AEs) by treatment course for the first three courses are summarized in Table 3. With the caveat that patients underwent cytoreductive therapy to reduce WBC below 20 K/μl before starting venetoclax and since venetoclax was started on day 2 of chemotherapy, tumor lysis syndrome was not observed. During course 1, grade 3/4/5 AE’s were febrile neutropenia (74%), ALT elevations (10%), AST elevations (4%), diarrhea (4%), other infection (6%), bilirubin elevation (2%), and rash (2%). All other AE’s in course 2 and 3 are listed in table 3. Early mortality was low with only one death (2%) within 4 weeks and 8 weeks. The only death within four weeks of induction occurred in a patient receiving a concomitant FLT3i. This patient died of bacteremia and sepsis prior to count recovery. There were two deaths of patients on study while in CR in consolidation cycles, both of whom had FLT3-mutated AML, receiving combined therapy with a FLT3i. Both of these patients died of infectious complications while cytopenic mid-course of treatment.
Table 3:
Adverse Events By Treatment Course
| Worst Grade AEs by Treatment Course | ||||
|---|---|---|---|---|
| During Course 1 (n=50) | ||||
| AE / Grade | G1 | G2 | G3 | G4 |
| Allergic rhinitis | 1 (2%) | |||
| ALT, SGPT | 23 (46%) | 6 (12%) | 4 (8%) | 1 (2%) |
| Anorexia | 2 (4%) | |||
| AST, SGOT | 6 (12%) | 2 (4%) | ||
| Bilirubin | 2 (4%) | 2 (4%) | 1 (2%) | |
| Bleeding other | 3 (6%) | |||
| Colitis | 1 (2%) | |||
| Constipation | 3 (6%) | |||
| Cough | 5 (10%) | |||
| Creatinine | 3 (6%) | |||
| Diarrhea | 11 (22%) | 2 (4%) | ||
| Dizziness | 2 (4%) | |||
| Edema, head & neck | 1 (2%) | |||
| Fatigue | 27 (54%) | |||
| Febrile Neutropenia | 2 (4%) | 37 (74%) | ||
| Fluid Overload | 1 (2%) | |||
| Hypokalemia | 3 (6%) | |||
| Hypophosphatemia | 1 (2%) | |||
| Infection-Other | 1 (2%) | 1 (2%) | 2 (4%) | |
| Insomnia | 2 (4%) | |||
| Mood alteration/anxiety | 1 (2%) | |||
| Mucositis | 16 (32%) | 1 (2%) | ||
| Nausea | 13 (26%) | |||
| Other | 1 (2%) | 1 (2%) | ||
| Otitis | 2 (4%) | |||
| Pain | 11 (22%) | |||
| Peri-orbital pain | 1 (2%) | |||
| Petechiae/purpura/bruising | 2 (4%) | |||
| Pruritis/Itching | 1 (2%) | |||
| Rash/desquamation | 13 (26%) | 1 (2%) | ||
| Seizure | 1 (2%) | |||
| Thrombosis/thrombus/embolism | 3 (6%) | |||
| Tinnitus | 1 (2%) | |||
| Vomiting | 2 (4%) | |||
| Weight loss | 1 (2%) | |||
| During Course 2 (n=44) | ||||
| Acidosis | 1 (2%) | 1 (2%) | ||
| ALT, SGPT | 4 (9%) | 1 (2%) | 1 (2%) | 6 (14%) |
| AST, SGOT | 1 (2%) | 1 (2%) | ||
| Bilirubin | 1 (2%) | 1 (2%) | ||
| Blurriness | 1 (2%) | 1 (2%) | ||
| Cough | 2 (5%) | 2 (5%) | ||
| Diarrhea | 2 (5%) | 2 (5%) | ||
| Dyspnea | 1 (2%) | 1 (2%) | ||
| Fatigue | 2 (5%) | 2 (5%) | ||
| Febrile Neutropenia | 8 (18%) | 8 (18%) | ||
| Fistula, GI | 1 (2%) | 1 (2%) | ||
| Infection-Other | 2 (5%) | 2 (5%) | ||
| Mucositis | 1 (2%) | 1 (2%) | ||
| Nausea | 2 (5%) | 2 (5%) | ||
| Other | 1 (2%) | 1 (2%) | ||
| Pain | 2 (5%) | 2 (5%) | ||
| Petechiae/purpura/bruising | 2 (5%) | 2 (5%) | ||
| Respriatory Distress | 1 (2%) | 1 (2%) | ||
| Syncope | 1 (2%) | 1 (2%) | ||
| Vomiting | 2 (5%) | 2 (5%) | ||
| During Course 3 (n=18) | ||||
| ALT, SGPT | 1 (6%) | |||
| AST, SGOT | 1 (6%) | |||
| Febrile Neutropenia | 2 (11%) | |||
| Other | 1 (6%) | |||
| Pain | 1 (6%) | |||
DISCUSSION
Sequential efforts have been made over the years to optimize AML therapy to improve the outcome of patients with newly diagnosed AML. Since CR is strongly associated with superior survival, producing higher rates of remission safely during induction can be achieved in several ways. These include higher doses of cytarabine, optimizing the anthracycline, and in some cases, adding a third chemotherapy drug. In the current study, we built on these modifications by adding venetoclax to enhance the anti-leukemic efficacy. Here, we report our experience with CLIA combined with venetoclax as a frontline treatment for newly diagnosed AML and high-risk MDS patients ≤ 65 years of age, demonstrating high rates of durable CR that translated into promising rates of EFS and OS. We observed high CR/CRi rates across ELN and prognostic risk groups. While patients with diploid and intermediate-risk cytogenetics experienced the highest response rates and most favorable outcomes, venetoclax plus CLIA was also associated with improved outcomes among patients with adverse-risk/complex cytogenetics when compared with contemporary analyses of intensive chemotherapy in this cytogenetic subgroup(22). Notably, 37 of 45 (82%) responding patients achieved MRD negativity, and 29 (62%) were able to proceed to alloSCT in first remission.
Based on its activity in combination with lower intensity therapy, venetoclax has become part of the standard of care for older / unfit patients with newly diagnosed AML. The VIALE-A study demonstrated a significantly improved composite complete remission (66.4% vs. 28.3%; p<0.001) and median OS (14.7 vs 9.6 months; p<0.001) with the combination of 5-azacitidine and venetoclax compared to 5-azacitidine alone(15). Recently, Chua et al. described their experience with venetoclax combined with the “5+2” regimen in older AML patients fit for intensive therapy. Among 51 patients (median age 72 years; range 63-80 years) treated with idarubicin (12 mg/m2 on D2-3), cytarabine (100 mg/m2 on D1-5), and escalating doses of venetoclax (50-600 mg on D1-14), the CR/CRi rate was 72% in the entire cohort and 97% in de novo AML. The median OS for the entire cohort was 11.2 months, and 31.3 months in de novo AML (22). Our approach was to incorporate venetoclax into a high-dose cytarabine based regimen among newly diagnosed younger and fit patients to maximize their response rates.
The remission rates and survival of patients receiving venetoclax plus CLIA compare favorably when taken in the context of published studies of intensive chemotherapy in younger and fit patients with AML. Among 657 patients with a median age of 48 years, Fernandez et. al. reported a CR rate 71% and median OS of 23.7 months with 7+3 using daunorubicin dose intensification to 90 mg/m(6). Willemze et al used higher dose cytarabine as part of induction for younger patients with AML. Among patients < 46 years, they reported a CR rate of 82.4%, 6-year EFS rate of 43.6%, 6-year OS rate of 51.9%(1). In the RATIFY study, the addition of midostaurin to 7+3 produced a CR rate of 58.9% and a median EFS and OS of 8.2 and 74.7 months, respectively among FLT3-mutated AML patients(23). In our small subset of FLT3-mutated patients treated with CLIA+venetoclax+FLT3i, we observed a CR/CRi rate of 89% with 12-month EFS and OS rates of 50.5 and 63.5%, respectively. Overall, the addition of venetoclax improved CR/CRi rate to 94% with 82% of patients achieving MRD negativity.
One of the primary concerns when adding venetoclax to intensive chemotherapy was the potential for increased myelosuppression that could go hand-in-hand with improved efficacy. Acknowledging this, we implemented a modified venetoclax dosing of 7 days (different from the on-label 28-day dosing) per course. The goal was to maximize the potentiation of BCL2 inhibition during the period of intensive chemotherapy and allow sufficient time for marrow recovery. The results were encouraging. The regimen was well tolerated with a low early mortality. The time to count recovery after induction was within 4-weeks, similar to other intensive regimens. Grade 3/4 AEs occurring with Venetoclax plus CLIA were primarily infectious complications. Febrile neutropenia accounted for the majority of the grade 3/4 AEs. The addition of a FLT3i in this regimen appeared to prolong myelosuppression, and the only death during induction was in a patient receiving a concomitant FLT3i. There were also three deaths in CR, two of which were patients who received an added FLT3i.
Our study has some limitations that should be noted. First, it is a single-center, single-arm phase II trial without comparator or control arm. However, it represents on of the first and largest cohort of well-annotated frontline AML patients with the goal of evaluating the efficacy and tolerability of venetoclax combined with high-dose cytarabine based therapy. The study can provide the basis for future randomized comparisons to help confirm the benefit in improving long term OS. While our median follow-up time on study is over a year (13.5 months), longer follow-up time is needed to confirm the durability of the responses and long term survival that we have observed.
In summary, venetoclax plus CLIA represents a safe and feasible intensive regimen for newly diagnosed patients with AML. From our single-center experience we observed high rates of durable MRD negative remissions and encouraging EFS and OS across all prognostic subgroups. Larger number of patients and multi-institutional studies of the CLIA-venetoclax regimen are warranted to confirm the possible benefits and safety of the regimen.
Supplementary Material
Research in context.
Evidence before this study:
We reviewed the literature reporting on modifications to intensive chemotherapy in newly diagnosed AML as well as the combination of venetoclax to lower intensity therapy in older and unfit patients with AML. A formal literature search was not conducted. Anthracycline and cytarabine dose intensification during induction therapy for younger adults with AML have led to improved outcomes. The Polish Adult Leukemia Group has shown that the addition of cladribine to the ‘7+3’ regimen has further improved survival in newly diagnosed AML. We previously incorporated cladribine with idarubicin and high-dose cytarabine in the CLIA regimen for frontline induction and consolidation therapy in younger patients with AML observing an composite complete remission rate of 81%. Recently, the addition of venetoclax has shown improved survival in older adults with AML when combined with low intensity hypomethylating agents. However, the safety and efficacy of adding venetoclax to intensive therapy in younger patients is not known.
Added value of this study:
These initial results are the first experience of adding venetoclax to the high-dose cytarabine-based CLIA regimen for newly diagnosed AML/MDS in younger patients fit to receive intensive therapy.
Implications of all the available evidence:
This intensive regimen of venetoclax combined with CLIA is highly active and produces high rates of minimal residual disease negative remission and encouraging rates of event-free and overall survival. This data supports the addition of venetoclax to intensive chemotherapy induction regimens and should be confirmed in phase 3 testing.
Funding/Support:
This trial was supported by M.D. Anderson Cancer Center Leukemia SPORE, P50CA100632, and M.D. Anderson Cancer Center Support Grant, P30CA016672, both by the NIH/NCI. Dr. Reville is supported by a T32 training grant from the NIH (T32CA009666).
Role of the Funding Source:
The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: Dr. Kadia reports grants from: Amgen, Ascentage, Astellas, AstraZeneca, BMS, Cellenkos, Pulmotech, Genfleet; Personal fees from: Agios, Cure, Daichi Snkyo, Genzyme, Liberum, Novartis, Sanofi-Aventis; and grants and personal fees from: Abbvie, Genetech, Jazz Pharmaceuticals, Pfizer. Dr. Borthakur reports grants from: Oncoceutics, Xbiotech USA, Arvina, Polaris, AstraZeneca, BMS, Cyclacel, GlaxoSmithKline, Janssen, Incyte, AbbVie; personal fees from: Argenx, PTC Therapeutics, BioTheryX, Nkarta, Inc, Treadwell Therapeutics, Curio Science; and grants and personal fees from: FTC Therapeutics, BioLine Rx, and Novartis. Dr. Yilmaz reports research support from Daiichi-Sankyo and Pfizer. Dr. DiNardo reports Research Support from: Calithera, Cleave, Jazz, Loxo and Personal fees from: Aprea, Cleave, Novartis, Takeda, Notable Labs; and grants and personal fees from: Abbvie, Agios, ImmuneOnc, Celgene/BMS, Daiichi Sankyo. Dr. Daver reports Research support from NOHLA, Glycomimetics, Sobi, Hanmi, Forty Seven, Newave, Trovagene, Covance, FATE, Novimmune and Personal fees from: Otsuka, Celgene, Incyte, Jazz Pharmaceuticals, Immunogen, Agios, Syndax, Trillium; and grants and personal fees from: Pfizer, BMS, Novartis, Daiichi-Sankyo, Karyopharm, Incyte, Abbvie, Genetech, Immunogen, Astellas, Servier, Gilead, Amgen, Sunesis. Dr. Jain reports grants from: Pfizer, Incyte, Aprea Therapeutics, Fate Therapeutics, Kite; personal fees from: Janssen, Beigene, TG Therapeutics; and grants and personal fees from: Pharmacyclics, AbbVie, Genentech, AstraZeneca, BMS, ADC Therapeutics, Cellectis, Adaptive Biotechnologies, Servier, Precision Biosciences. Dr. Pemmaraju reports grants from: Affymetrix, SagerStrong Foundation, Samus Therapeutics, Cellectis, Daiichi Sankyo, Plexxikon; personal fees from: Pacylex Pharmaceuticals, ImmunoGen, BMS, Blueprint Medicines, Incyte, LFB Biotechnologies, Celgene, AbbVie, MustangBio, Roche Diagnostics, DAVA Oncology, Springer Science + Business Media LLC; and grants and personal fees from: AbbVie, Stemline Therapeutics, and Novartis. Dr. Konopleva reports grants from Ablynx, Agios, Ascentage, Astra Zeneca, Rafael Pharmaceutical, Sanofi; personal fees from Reata Pharmaceutical, Janssen; and grants and personal fees from Abbvie, F. Hoffman La-Roche, Stemline Therapeutics, Forty-Seven, Genetech. In addition, Dr. Konopleva has a patent US 7,795,305 B2 CDDO-compounds and combination therapie with royalties paid to Reata Pharm., a patent Combination Therapy with a mutant IDH1 Inhibitor and a BCL-2 licensed to Eli Lilly, and a patent 62/993,166 combination of a mcl-1 inhibitor and midostaurin, uses and pharmaceutical compositions thereof pending to Novartis. Dr. Ravandi reports grants from AbbVie. Dr. Kantarjian reports grants from: Ascentage, BMS, Daiichi-Sankyo, Immunogen, Jazz Pharmaceuticals, Sanofi; personal fees from: Actinium, Adaptive Biotechnologies, Apptitude Health, BioAscend, Daiichi-Sankyo, Delta Fly, Janssen Global, Novartis, Oxford Biomedical, Takeda Oncology, and grants and personal fees from: Abbvie, Amgen, Pfizer. All other authors report no conflicts of interest.
Prior presentations: ASCO 2020, EHA 2020, and ASH 2020
Data sharing: Qualified researchers may request access to individual patient-level data reported in this Article after print publication of the current Article. No identifying data will be provided. All requests for data must include a description of the research proposal and be submitted to the corresponding author. The study protocol, including the statistical analysis plan, has been included in the appendix.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Willemze R, Suciu S, Meloni G, Labar B, Marie J-P, Halkes CJM, et al. High-Dose Cytarabine in Induction Treatment Improves the Outcome of Adult Patients Younger Than Age 46 Years With Acute Myeloid Leukemia: Results of the EORTC-GIMEMA AML-12 Trial. Journal of Clinical Oncology. 2014;32(3):219–28. [DOI] [PubMed] [Google Scholar]
- 2.Löwenberg B, Pabst T, Vellenga E, van Putten W, Schouten HC, Graux C, et al. Cytarabine Dose for Acute Myeloid Leukemia. New England Journal of Medicine. 2011;364(11):1027–36. [DOI] [PubMed] [Google Scholar]
- 3.Li W, Gong X, Sun M, Zhao X, Gong B, Wei H, et al. High-dose cytarabine in acute myeloid leukemia treatment: a systematic review and meta-analysis. PLoS One. 2014;9(10):e110153–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kantarjian H, Kadia T, DiNardo C, Daver N, Borthakur G, Jabbour E, et al. Acute myeloid leukemia: current progress and future directions. Blood Cancer Journal. 2021;11(2):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Löwenberg B, Ossenkoppele GJ, van Putten W, Schouten HC, Graux C, Ferrant A, et al. High-dose daunorubicin in older patients with acute myeloid leukemia. N Engl J Med. 2009;361(13):1235–48. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez HF, Sun Z, Yao X, Litzow MR, Luger SM, Paietta EM, et al. Anthracycline dose intensification in acute myeloid leukemia. N Engl J Med. 2009;361(13):1249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burnett AK, Russell NH, Hills RK, Kell J, Cavenagh J, Kjeldsen L, et al. A randomized comparison of daunorubicin 90 mg/m2 vs 60 mg/m2 in AML induction: results from the UK NCRI AML17 trial in 1206 patients. Blood. 2015;125(25):3878–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devillier R, Bertoli S, Prébet T, Huguet F, Etienne A, Charbonnier A, et al. Comparison of 60 or 90 mg/m(2) of daunorubicin in induction therapy for acute myeloid leukemia with intermediate or unfavorable cytogenetics. Am J Hematol. 2015;90(2):E29–30. [DOI] [PubMed] [Google Scholar]
- 9.Mandelli F, Vignetti M, Suciu S, Stasi R, Petti M-C, Meloni G, et al. Daunorubicin versus mitoxantrone versus idarubicin as induction and consolidation chemotherapy for adults with acute myeloid leukemia: the EORTC and GIMEMA Groups Study AML-10. J Clin Oncol. 2009;27(32):5397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pautas C, Merabet F, Thomas X, Raffoux E, Gardin C, Corm S, et al. Randomized study of intensified anthracycline doses for induction and recombinant interleukin-2 for maintenance in patients with acute myeloid leukemia age 50 to 70 years: results of the ALFA-9801 study. J Clin Oncol. 2010;28(5):808–14. [DOI] [PubMed] [Google Scholar]
- 11.Gandhi V, Estey E, Keating MJ, Plunkett W. Fludarabine potentiates metabolism of cytarabine in patients with acute myelogenous leukemia during therapy. J Clin Oncol. 1993;11(1):116–24. [DOI] [PubMed] [Google Scholar]
- 12.Holowiecki J, Grosicki S, Robak T, Kyrcz-Krzemien S, Giebel S, Hellmann A, et al. Addition of cladribine to daunorubicin and cytarabine increases complete remission rate after a single course of induction treatment in acute myeloid leukemia. Multicenter, phase III study. Leukemia. 2004;18(5):989–97. [DOI] [PubMed] [Google Scholar]
- 13.Holowiecki J, Grosicki S, Giebel S, Robak T, Kyrcz-Krzemien S, Kuliczkowski K, et al. Cladribine, but not fludarabine, added to daunorubicin and cytarabine during induction prolongs survival of patients with acute myeloid leukemia: a multicenter, randomized phase III study. J Clin Oncol. 2012;30(20):2441–8. [DOI] [PubMed] [Google Scholar]
- 14.Jain P, Kantarjian HM, Ravandi F, Jabbour E, Daver N, Pemmaraju N, et al. Cladribine Combined with Idarubicin and Ara-C (CLIA) As a Frontline and Salvage Treatment for Young Patients (≤65 yrs) with Acute Myeloid Leukemia. Blood. 2016;128(22):1639-. [Google Scholar]
- 15.DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N Engl J Med. 2020;383(7):617–29. [DOI] [PubMed] [Google Scholar]
- 16.Wei AH, Montesinos P, Ivanov V, DiNardo CD, Novak J, Laribi K, et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: a phase 3 randomized placebo-controlled trial. Blood. 2020;135(24):2137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DiNardo CD, Maiti A, Rausch CR, Pemmaraju N, Naqvi K, Daver NG, et al. 10-day decitabine with venetoclax for newly diagnosed intensive chemotherapy ineligible, and relapsed or refractory acute myeloid leukaemia: a single-centre, phase 2 trial. The Lancet Haematology. 2020;7(10):e724–e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel KP, Ruiz-Cordero R, Chen W, Routbort MJ, Floyd K, Rodriguez S, et al. Ultra-Rapid Reporting of GENomic Targets (URGENTseq): Clinical Next-Generation Sequencing Results within 48 Hours of Sample Collection. The Journal of molecular diagnostics : JMD. 2019;21(1):89–98. [DOI] [PubMed] [Google Scholar]
- 19.Xu J, Jorgensen JL, Wang SA. How Do We Use Multicolor Flow Cytometry to Detect Minimal Residual Disease in Acute Myeloid Leukemia? Clin Lab Med. 2017;37(4):787–802. [DOI] [PubMed] [Google Scholar]
- 20.Cheson BD, Bennett JM, Kopecky KJ, Büchner T, Willman CL, Estey EH, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21(24):4642–9. [DOI] [PubMed] [Google Scholar]
- 21.Thall PF, Simon RM, Estey EH. Bayesian sequential monitoring designs for single-arm clinical trials with multiple outcomes. Statistics in Medicine. 1995;14(4):357–79. [DOI] [PubMed] [Google Scholar]
- 22.Chua CC, Roberts AW, Reynolds J, Fong CY, Ting SB, Salmon JM, et al. Chemotherapy and Venetoclax in Elderly Acute Myeloid Leukemia Trial (CAVEAT): A Phase Ib Dose-Escalation Study of Venetoclax Combined With Modified Intensive Chemotherapy. J Clin Oncol. 2020;38(30):3506–17. [DOI] [PubMed] [Google Scholar]
- 23.Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N Engl J Med. 2017;377(5):454–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.