Abstract
Mutations in protein kinases are often associated with the development of cancer, and application of mutant-specific inhibitors as therapeutic measures have shown a remarkable improvement in prolonging patient survival. However, it has also been observed that tumors bearing certain mutation types are more resistant to current approved drugs. Importantly, many resistant mutations are located in regions outside substrate or inhibitor binding sites, indicating allosteric effects. Understanding how mutations trigger effects over a distant site of the protein requires a deeper investigation of the molecular origin of allosteric regulation networks in kinases. In this chapter, we show the application of single-molecule optical tweezers to selectively manipulate specific regions of proteins to trace allosteric signals, thereby allowing the elucidation of allosteric communication networks. We illustrate this approach using as model system the regulatory subunit of protein kinase A. This single-molecule optical tweezers approach, however, can be readily applicable to study other kinases, and can be further expanded to screen potential allosteric drugs for future therapeutics.
Keywords: Allostery, Kinases, Single molecule optical tweezers, Mechanical fingerprints, DNA-protein covalent attachment, Selective manipulation, Force-extension curves
1. Introduction
Protein kinases regulate diverse cellular processes through their ability to activate downstream signaling proteins. Deregulation of kinases signaling caused by mutations is commonly found in human diseases, including cancer [1, 2]. For example, mutations in BRAF are associated with melanomas, papillary thyroid cancers, non–small-cell lung cancer, and leukemias [3–6]. Mutations in protein kinase A (PKA) causes the Carney complex diseases and acrodysostosis [7, 8]. Mutations within KRAS has been found associated with pancreatic cancer, colorectal cancer, and lung adenocarcinoma [9–11]. Drugs that target specific mutations have been approved by the FDA, and showed a remarkable improvement in treatment, such as vemurafenib and dabrafenib that targets BRAF harboring the mutations V600E/K [12, 13]. However, it has been discovered that tumors bearing mutations other than V600 are less sensitive to the approved drugs [1]. In order to understand how specific mutations can affect distal active sites, regulatory sites, or drug efficiencies, it is imperative a deeper investigation on the molecular origin of the allosteric regulation of protein kinases (Fig. 1a).
Fig. 1.

(a) Schematic of the allosteric effects in kinases caused by mutations or inhibitor binding. (b) Schematic representation of the optical tweezers experimental setup. The target protein is tethered between two polystyrene beads. The streptavidin bead (SA) is held by the suction from a micropipette tip and the top anti-digoxigenin bead (AD) is trapped in the optical trap. The target protein contains two cysteines that allow for the covalent attachment of dsDNA handles via disulfide bonds. The dsDNA is attached to the polystyrene beads through biotin–streptavidin and digoxigenin–anti-digoxigenin interactions. (c) Effect of external forces on light patterns leaving a trap. (top) At zero force: laser beam enters through the left objective lens and the bead is trapped at the focal point. The laser beam enters the right objective lens and is projected onto a position detector surface. Dotted lines define the numerical aperture of the lens. (bottom) A force from above pushes the bead, resulting a shift of the laser beam downward. Pattern projected on detector is offset down allowing to measure the change in position. See ref. [30] for instrumental details
The catalytic activity of protein kinases is typically regulated by the allosteric control of regulatory domains. These regulatory domains respond to specific ligands, cofactors, or posttranslational modifications, resulting in conformational transitions between inactive and active states. Optical tweezers is a powerful force spectroscopy technique used to investigate the effect of mechanical forces on single biomolecules [14–17]. In optical tweezers experiments, the individual biomolecule of interests is tethered between two polystyrene beads or between one bead and a glass slide. Figure 1b shows the schematic representation of a protein tether formed between two beads [18, 19]. One polystyrene bead is fix in a single position by suction using a micropipette, whereas the other bead is stably trapped by the laser. Tethering a biomolecule is achieved by the covalent attachment of molecular handles, such as double-stranded DNA. Hence, forces can be mechanically applied to the protein system by moving the bead in the optical trap away or toward the bead on the micropipette tip. Recent techniques in single molecule optical tweezers have shown that the conformation or the folding state of a protein can be probed by pulling the biomolecule with an applied force [20–22]. This mechanical manipulation at the single molecule level can provide unique signatures of each conformation, revealing a relationship between conformation and force, and thus can served as unique “mechanical fingerprints” [23, 24]. By selectively manipulating different parts of a protein kinase, it is possible. to follow allosteric communication and signals transduced through the protein structure [14, 25, 26]. With a complete allosteric network mapped out by mechanical manipulation, the mechanism associated with inhibitor binding or disease mutations can be traced, providing a direct avenue to screen potential drugs for clinical therapeutics.
In this chapter, we illustrate the selective manipulation of the regulatory subunit (RIα) of PKA as a model system. The activation of the catalytic subunit of PKA is allosterically regulated by the binding of cAMP to RIα [27–29]. Here, we show the dissection of allosteric networks in PKA-RIα by revealing the mechanical fingerprints in the two cAMP binding domains (termed CNB-A and -B domains) in the activation process. The purpose of this chapter is to provide a detailed description onto how to prepare protein kinase samples that can be readily applicable for single molecule optical tweezers experiments.
2. Materials
2.1. Optical Tweezer Apparatus
All optical tweezers parts are purchased according to the list from TweezersLab website (http://tweezerslab.unipr.it/cgi-bin/mt/home.pl), which is based on an opposed-beam, single trap optical tweezers design [30]. The instrument is assembled and calibrated by Steven Smith from Steven B. Smith Engineering.
2.2. Microfluidic Chamber
Microfluidic chambers can be fabricated according to the template provided on the TweezersLab website: (tweezerslab.unipr.it/cgi-bin/mt/documents.pl/Show?=ida77c&sort!DEFAULT&search=&hits=20.) Microfluidic chambers used this chapter are purchased from Steven B. Smith Engineering.
-
Syringes: BD 10 mL syringe eccentric tip (REF 305482).
BD 1 mL syringe slip tip (REF 309659)
-
Tubing: BD Intramedic polyethylene tubing PE10 (Catalog no. 427401).
BD Intramedic polyethylene tubing PE10 (Catalog no. 427401).
2.3. Beads Preparation
Streptavidin beads (Spherotech, SVP-20-5, 0.5% w/v, 5 mL).
Protein G-coated beads (Spherotech, PGP-30-5, 0.5% w/v, 5 mL).
AbX buffer: 100 mM Na2HPO4, 100 mM NaCl.
Anti-digoxigenin sheep polyclonal antibody (Roche: 11333089001, 200 μg) is dissolved in 200 μL PBS buffer resulting a final concentration of 1 μg/μL. The excess can be stored at −80 °C for future use.
Dimethyl pimelimidate (DMP, Thermo Fisher Pierce: 21666, 50 mg) is dissolved in 1 mL of AbX buffer.
DNA cross-linking buffer: 50 mM Tris, 100 mM NaCl, pH 7.6.
2.4. Sample Preparation for Optical Tweezers Samples
Annealing Buffer: 10 Mm Tris, 10 Mm EDTA, 50 Mm NaCl, pH 7.4.
10× ligation buffer: 400 Mm Tris–HCl, 100 Mm MgCl2, and 5 Mm ATP, pH 7.8.
2.5. Oligo Sequence for Double-Stranded DNA Handles
All oligos are purchased from Integrated DNA Technologies Inc.
-
dsOligos,
Both dsOligos shared the same thiol-modified forward sequence: 5′-Thiol- GTTACGCCTATTCCTATCATATGAAGACA (IDT Inc).
Reverse sequence for Restriction site 1 (RS1) is 5′-Phosphate-CGGAGTGTCTTCATATGATAGGAATAGGCGTAAC.
Reverse sequence for Restriction site 2 (RS2) is 5′-Phosphate-CGACGTGTCTTCATATGATAGGAATAGGCGTAAC.
-
370 bp-long DNA handles.
For dsOligos labeled RS1 in the protein–oligo chimera:
Forward primer 5′-TATTATTTTCTCCCATGAAGACGGTCCGCGACTG.
Reverse primer 5′-Biotin- CGGTATCGTCGTATCCCACTACC.
For dsOligos labeled RS2 in the protein–oligo chimera:
Forward primer 5′-GACGATACCGAAGACAGGTCGTGTTATATCC.
Reverse primer 5′-digoxigenin- CCGTGCAGTCGATGATAAGCTGTC.
3. Methods
3.1. Microfluidic Chambers
The microfluidic chamber for optical tweezers measurement is held in-between two objective lenses and consists of three channels (Fig. 2a, b). The center channel is connected by two dispenser tubes to the top and bottom channels. A fixed micropipette exists in the center channel and holds the streptavidin beads via vacuum suction by a tubing-connected syringe. The laser trap is focused by the two objective lenses and traps the anti-digoxigenin coated beads in the center channel where the measurements are performed. The picture of the view in the center channel is shown in Fig. 2c.
Fig. 2.
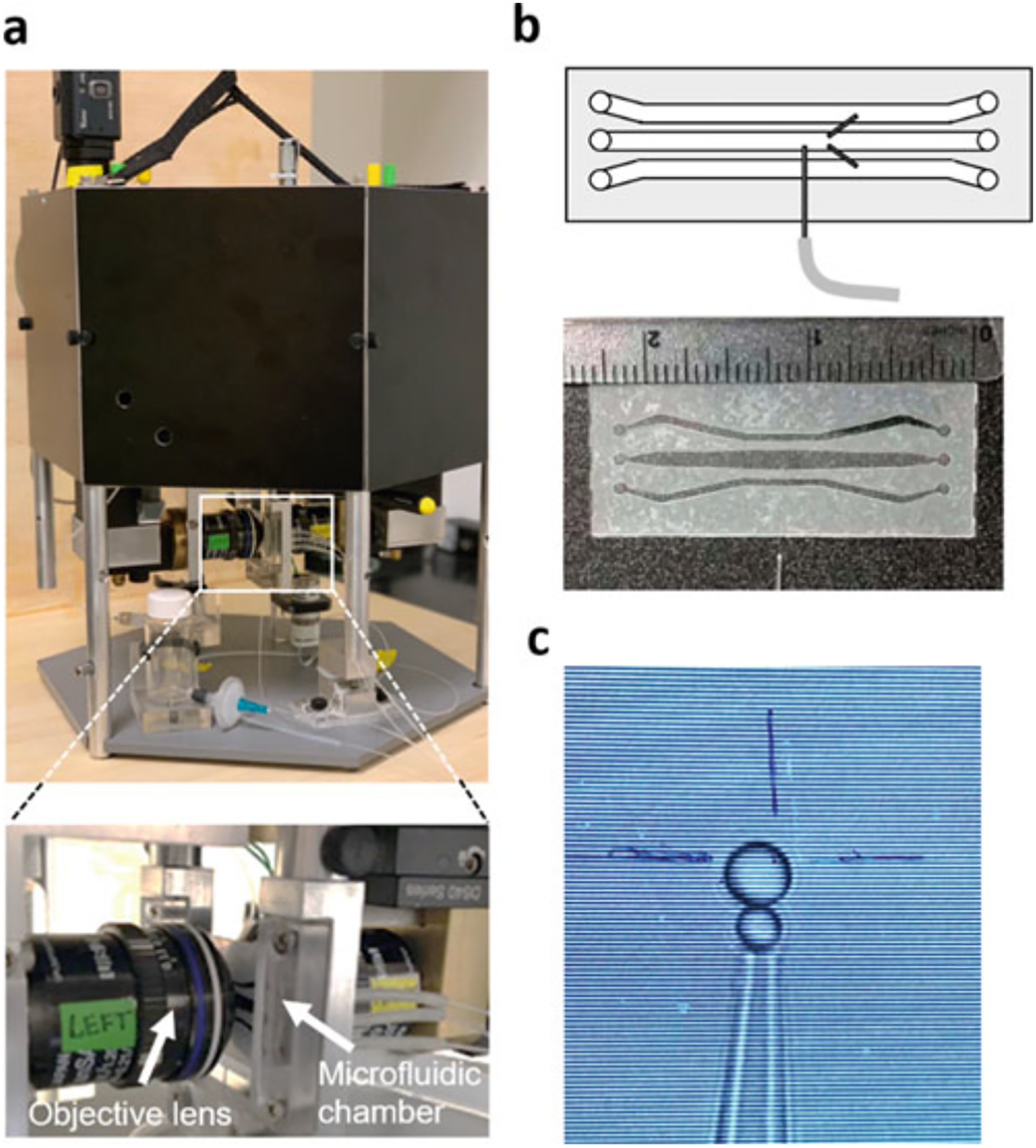
(a) Photo of MiniTweezers. Zoom in: A microfluidic chamber inserted between two objective lenses in optical tweezers instrument. (b) Schematic of the microfluidic chambers that consist of three channels. Two dispenser tubes connecting the top and bottom channel to the central channel, respectively. A micropipette is placed in the central channel for the streptavidin bead suction. A photo of the microfluidics showing the dimension of the microfluidic chambers. (c) An anti-digoxigenin bead with a diameter of ~3 μm is in the proximity to a streptavidin bead with the diameter of 2 μm visualized on the monitor screen during the optical tweezers measurement
3.2. Bead Preparation
Streptavidin-coated (SA) polystyrene beads with a diameter of 2.1 μm are directly purchased from Spherotech.
Anti-digoxigenin–coated (AD) beads are prepared from protein G-coated polystyrene beads with a diameter between 3.0 and 3.4 μm (see Note 1). Firstly, protein G-coated beads are resuspended for 30 min by vortexing the bottle at minimum speed. 1 mL of resuspended beads are spun down at 3000 rpm for 30 min and the supernatant is removed. The beads are then resuspended in 1 mL AbX buffer and mixed with 65 μL of anti-digoxigenin sheep polyclonal antibody solution and 30 μL DMP solution to the beads. The mixture is vortexed at the minimum speed at room temperature for 1 h. The beads are spun down at 4000 rpm for 10 min and the supernatant is removed. The beads are resuspended in 1 mL of 1 M Tris base buffer and are further vortexed at the minimum speed at room temperature for 2 h. After spinning down at 4000 rpm for 10 min and removing the supernatant, the beads are resuspended in 1 mL of DNA cross-linking buffer. To minimize stickiness, the beads are passivated with BSA by the following steps (see Note 2): The beads are washed with 500 μL of DNA cross-linking buffer three times, the spun down at 4000 rpm for 10 min to remove the supernatant. Incubate the beads with 1 mL of 10× BSA (1 mg/mL) for 30 min. The mixture is spun down at 4000 rpm for 10 min and the beads are resuspended with 500 μL DNA cross-linking buffer. Repeat washing step two times. Finally, resuspend the beads in 900uL DNA cross-linking buffer and store at 4 °C.
3.3. Sample Preparation for Optical Tweezers Measurement
3.3.1. Protein Construct Design
Two cysteine mutations are introduced at the residue positions that do not disrupt the properties, binding affinity, or activity of the protein (see Note 3). Any intrinsic cysteines that are solvent exposed need to be mutated to avoid unspecific double-stranded DNA handle attachment (see Note 3). In the regulatory subunit of PKA, the mutations C345A and C360A are introduced in the CNB-B domain to prevent undesired reactions with the thiol-modified dsDNA handles. To manipulate each individual CNB domain (termed Type-I constructs) we introduce the mutations S110C/M243C and M243C/S376C for the isolated CNB-A and CNB-B domains, respectively. To selectively manipulate either the CNB-A domain or the CNB-B domain (termed Type-II constructs) we introduce into the regulatory subunit the mutations S110C/M243C and M243C/S376C, respectively.
All the protein constructs are expressed in BL21(DE3) (NEB) and purified as described previously [22, 28, 31]. Briefly, the protein is expressed in BL21(DE3) competent cells overnight at 18 °C with 1 mM IPTG. The cells are lysed in lysis buffer (20 mM MES, 100 mM NaCl, 2 mM EGTA, 2 mM EDTA, 5 mM DTT, pH 6.5) and the spun-down supernatant is precipitated with 40% ammonium sulfate before binding to a homemade cAMP-coupled agarose resin. The protein is eluted from the cAMP-coupled resin with cGMP (20 mM cGMP in lysis buffer) and run on a size exclusion column to remove the excess cGMP and aggregated proteins. The protein is stored in gel filtration buffer (50 mM MES, 200 mM NaCl, 2 mM EGTA, 2 mM EDTA 5 mM DTT, pH 5.8) before use.
3.3.2. Integrated Method to Attach dsDNA Handles and Select Functional Proteins
-
Thiol-Modified dsOligo Preparation.
Two sets of modified double stranded oligos (dsOligos) with a thiol group in the 5′ end of the forward sequence, and a 5′ phosphate in the reverse complementary sequence (IDT, Inc.). Both dsOligos shared the same thiol-modified forward sequence: 5′-Thiol- GTTACGCCTATTCCTATCATATGAAGACA. However, the reverse complementary sequence had unique, nonpalindromic 5′ overhangs (underlined) referred as RS1 (5′-Phosphate-CGGA GTGTCTTCATATGATAGGAATAGGCGTAAC) and RS2 (5′-Phosphate-CGACGTGTCTTCATATGATAGGAATAGGCGTAAC) for restriction sites 1 and 2, respectively.
Thiol- and phosphate-modified oligos are dissolved in annealing buffer and annealed (95 °C to 4 °C, 1 °C/min) in a Mastercycler Nexus GX2 (Eppendorf). The thiol groups of the dsOligos are deprotected in 0.17 M sodium phosphate (pH 8.0) with 40 mM dithiothreitol (DTT) overnight at 37 °C. The next day, deprotected dsOligos are purified using an Amersham PD-10 column. The purified dsOligos are concentrated by ethanol precipitation, dissolved in annealing buffer, and store at −20 °C with 40 mM DTT. A typical concentration of dsOligos is 2 mM.
-
DTDP-activation of cysteine-modified proteins.
The activation of the thiol-modified protein with 2,2′-dithioldipyridine (DTDP) followed the protocol described previously [32] with minor modifications. Briefly, prior to the covalently attachment of the dsOligos, the protein is reduced with 5 mM DTT overnight in DNA cross-linking buffer. The next day, the protein is concentrated to ~500 μM (see Note 4). The excess DTT is removed by three consecutive Micro Bio-Spin 6 chromatography columns (Bio-Rad) preequilibrated with DNA cross-linking buffer. Then, the protein is reacted with a 5–25 molar excess DTDP for 2 h at room temperature (see Note 5). The excess DTDP is removed using three additional Micro Biospin 6 column preequilibrated with DNA cross-linking buffer.
-
Protein–Oligo Attachment.
Both dsOligos are diluted to ~200 mM in DNA cross-linking buffer with 10 mM DTT, and incubated overnight at 37 °C. The excess of DTT is removed using three Micro Bio-Spin 6 chromatography columns. The resulting dsOligo concentration is measured spectrophotometrically, and then immediately reacted with the thiol-pyridine activated protein (Fig. 3a, step 1). The molar ratio of protein and the two dsOligos is 1:1:1 with a final concentration of ~100 μM each.The reaction is incubated at 4 °C overnight.
-
Functional selection of protein–oligo chimera.
The mixture of protein and dsOligos is incubated with a cAMP-coupled agarose resin for 4 h at 4 °C (Fig. 3a, step 2). Preparation of the resin is described by the Diller et al. [33]. The functional selection step allows for removing unreacted dsOligos while at the same time purifying proteins able to bind its natural ligand, namely, cAMP. To thoroughly remove the excess of unreacted dsOligos, the agarose resin is washed with DNA cross-linking buffer three times with at least 20 volumes of the resin. The protein–oligo chimera is eluted using increasing amounts of cAMP dissolved in DNA cross-linking buffer: 0.02 mM, 0.2 mM, 2 mM, and 20 mM (Fig. 3a, step 3). For each elution step, the solution is incubated for 30 min. All elutions containing the protein–oligo chimera are aliquoted and stored with 30% glycerol at −20 °C. Before the optical tweezers experiments, a small aliquot of protein–oligo chimera is directly ligated to dsDNA handles of variable lengths, tailored to a specific experimental geometry (Fig. 3a, step 4). Next, we describe the preparation of dsDNA handles of ~370 bp each (referred as long dsDNA handles) as an example.
-
Preparation of 370 bp-long dsDNA handles.
The biotin- and digoxigenin-modified dsDNA handles are generated by PCR reaction using the plasmid pPROEX-HTa (Addgene) as template. A 332 bp biotin-modified dsDNA handle is prepared using the forward primer 5′- TATTATTTTCTCCCATGAAGACGGTCCGCGACTG together with reverse primer 5′-Biotin-CGGTATCGTCGTATCCCACTACC (IDT, Inc) (see Note 6). This handle is ligated to the dsOligo labeled RS1 in the protein–oligo chimera. The 315 bp digoxigenin-modified dsDNA handle is generated using the forward primer 5′-GACGATACCGAAGACAGGTCGTGTTATATCC and reverse primer 5′-digoxigenin-CCGTGCAGTCGATGATAAGCTGTC(IDT, Inc). This handle is ligated to the dsOligo labeled RS2 in the protein–oligo chimera. The PCR products are purified using NucleoSpin Gel and PCR cleanup kits (Clontech Laboratories). Usually, 10–12 μg of dsDNA handles can be prepared at a time using 8 of 100 μL PCR reactions. The PCR product is further digested with BpiI enzyme (Thermo Scientific) at 37 °C overnight. BpiI recognizes the sequence GAAGAC (underlined in forward primers), however, it cuts 2 bp downstream, leaving a 4 bp nonpalindromic sticky overhang. Therefore, even if both dsDNA handles are generated from the same plasmid or DNA template, BpiI provides the possibility of having different overhang sequences. The plasmid pPROEX-HTa has two GAAGAC sequences, located ~350 bp from each other. The two primer sets used in this study are designed to amplify different segments of the template.
-
Ligation of dsDNA handles to protein–oligo chimera.
dsDNA handles can be directly ligated to the protein–oligo chimera. By using nonpalindromic overhang sequences there is no ligation between the handles themselves, which otherwise can make a tether in the optical tweezers and decreases the efficiency of data collection.
Usually, about 150 ng of each dsDNA handle is needed for a 20 μL ligation. We used a homemade 10× ligation buffer (see Note 7). Here, we find that the ideal ligation ratio is 1:1:1 for protein–oligo chimera and each handle.
Fig. 3.

(a) An integrated method to attach dsDNA handles and select for functional protein molecules. After its purification, the target protein is covalently linked with thiol-modified dsOligos (step 1). The protein–oligo chimera is functionally selected using a ligand-coupled resin (step 2). Functional and properly folded proteins are eluted using a concentration gradient of a competitor, generating different protein liganded states (step 3). Ligation of biotin- and digoxigenin-modified dsDNA handles (step 4). (b) Monitoring the dsDNA handle attachment reaction and functional selection of the CNB-A domain in the regulatory subunit of PKA by electrophoresis. (1) 5′ thiol-modified dsOligos are attached to the CNB-A. (2) The protein–oligo chimera is functionally selected and eluted from cAMP-coupled agarose using an increasing concentration of cAMP: 0.02 mM, 0.2 mM, 2 mM, and 20 mM. (3) The selected protein–oligo chimera is ligated with 370 bp dsDNA handles modified with digoxigenin (green square) and biotin (orange circle). All gels are native acrylamide gels stained with ethidium bromide
3.4. Optical Tweezers Measurement
Three channels in the microfluidics chamber are cleaned and flowed with DNA cross-linking buffer. 2 μL of SA beads (Spherotech) is diluted into 1 mL with DNA cross-linking buffer and is injected from the bottom channel. The laser focus traps the SA beads from the opening of the dispenser tube connecting the bottom and central channels and moves it close to the micropipette for suction. The ligation product is diluted ~1000-fold in DNA cross-linking buffer. We then take 1 μL of the diluted ligation product and mix it with 3 μL of 3.1 μm AD beads (Spherotech) for 5 min at room temperature. The sample is further diluted to 1 mL before applying it to the top channel of the microfluidic chamber. The AD beads are brought to SA beads in close proximity by laser focus to form the tether. The optical tweezer experiments are performed in DNA cross-linking buffer in a temperature-controlled room at 23 °C. All data is collected in a MiniTweezers instrument. Force-ramp data is collected at a sampling rate of 200 Hz and a pulling speed of 75 nm/s.
3.5. Example of Results
Typical force-extension curves collected by optical tweezers is shown in Fig. 4. The red curves show the unfolding process with a rip that corresponds to the unfolding events of the protein of interests. The blue curves indicate the refolding process. In the apo state, the isolated CNB-A domain unfolds at an average force of Favg ~ 8.8 pN while the isolated CNB-B domain unfolds at Favg ~ 7.3 pN. The presence of cAMP revealed important differences between the two CNB domains. The unfolding force of the isolated CNB-B domain increases to Favg = 12.0 pN, while for the isolated CNB-A domain, the average unfolding force increase to Favg = 17.4 pN. We studied interdomain or allosteric interactions triggered by cAMP using type-II constructs. We find that both CNB domains are stabilized by the presence of their counterpart when bound to the cyclic nucleotide. Interestingly, the magnitude of stabilization is asymmetric: The CNB-A domain stabilizes the CNB-B domain by an additional ~8 pN, resulting in Favg = 19.7 pN. The presence of the CNB-B domain induces a mechanical stabilization to the CNB-A domain of ~3 pN, resulting in Favg = 20.3 pN. Therefore, using optical tweezers, we found that the = cAMP-dependent communications between the CNB domains are bidirectional and asymmetric, highlighting a unique role for each domain in the activation mechanism of PKA (see Notes 8 and 9).
Fig. 4.

(a) Force-extension curves and unfolding force probability distributions for the CNB-B (top) and CNB-A (bottom) domains. Numbering corresponds to the isolated CNB domains in the apo (1) or cAMP-bound states (2), and selective unfolding of the CNB domains bound to cAMP (3)
4. Notes
Choosing a different size of SA and AD beads helps distinguishing the two bead types on the monitor screen.
The stickiness of AD beads can be further decreased by repeating the steps of the passivation with BSA.
Perform functional assays that ensure that mutations do not affect the wild type behavior of the protein. Assays could include kinase activation or inhibition, ligand binding, etc.
For the dsOligo attachment, higher protein concentration results in higher yield production of protein–dsOligo. However, depending on the properties of the protein of interest, the concentration of protein after passing six Micro Bio-Spin 6 can be as low as 5 μM.
2,2′-dithioldipyridine (DTDP) are prepared in acetonitrile. During the incubation of DTDP, a white cloud might form in the reaction due to the low solubility of DTDP in an aqueous solution. In general, this white cloud is not caused by the aggregation of the target protein.
The dsDNA handles can be designed and prepared with a flexible length that fits the experiment requirement.
The 100 mM DTT typically seen in commercial ligation buffers is omitted to prevent the reduction of the disulfide linkage between the protein and dsOligos.
The extension of the polypeptide chains subjected to external force follows the worm-like chain (WLC) model. Therefore, the WLC model can be implemented into optical tweezers measurement to estimate the number of amino acids participate in the unfolding events.
Kinetic and thermodynamic parameters, such as the lifetime of the folded protein, distance to the transition states, and free energy of the unfolding process, can be extracted from the obtained force distribution following the established studies [34–37].
Acknowledgments
This work was supported by NSF grant MCB1715572 (to R. A. M.) and National Institutes of Health grant 1R15GM135866 (to R. A. M.).
References
- 1.Karoulia Z, Gavathiotis E, Poulikakos PI (2017) New perspectives for targeting RAF kinase in human cancer. Nat Rev Cancer 17: 676–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts PJ, Der CJ (2007) Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 26(22):3291–3310. 10.1038/sj.onc.1210422 [DOI] [PubMed] [Google Scholar]
- 3.Akbani R et al. (2015) Genomic classification of cutaneous melanoma. Cell 161 (7):1681–1696. 10.1016/j.cell.2015.05.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kimura ET et al. (2003) High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res 63 (7):1454–1457 [PubMed] [Google Scholar]
- 5.(2014) Corrigendum: Comprehensive molecular profiling of lung adenocarcinoma. Nature. 10.1038/nature13879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tiacci E et al. (2011) BRAF mutations in hairy-cell leukemia. N Engl J Med 365 (10):960–961. 10.1056/NEJMoa1014209 [DOI] [PubMed] [Google Scholar]
- 7.Horvath A et al. (2010) Mutations and polymorphisms in the gene encoding regulatory subunit type 1-alpha of protein kinase a (PRKAR1A): an update. Hum Mutat 31 (4):369–379. 10.1002/humu.21178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruystens JG et al. (2016) Structure of a PKA RIα recurrent acrodysostosis mutant explains defective cAMP-dependent activation. J Mol Biol 428(24 Pt B):4890–4904. 10.1016/j.jmb.2016.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foster SA, Klijn C, Malek S (2016) Tissue-specific mutations in BRAF and EGFR necessitate unique therapeutic approaches. Trends Cancer 2(12):699–701. 10.1016/j.trecan.2016.10.015 [DOI] [PubMed] [Google Scholar]
- 10.Cerami E et al. (2012) The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2(5):401–404. 10.1158/2159-8290.CD-12-0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao J et al. (2013) Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 6(269):pl1. 10.1126/scisignal.2004088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hauschild A et al. (2012) Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 380:358–365 [DOI] [PubMed] [Google Scholar]
- 13.Chapman PB et al. (2011) Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 364: 2507–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shank EA, Cecconi C, Dill JW, Marqusee S, Bustamante C (2010) The folding cooperativity of a protein is controlled by its chain topology. Nature 465:637–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maillard RA et al. (2011) ClpX(P) generates mechanical force to unfold and translocate its protein substrates. Cell 145:459–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woodside MT, Block SM (2008) Folding in single riboswitch aptamers. Science 180: 2006–2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cecconi C, Shank EA, Bustamante C, Marqusee S (2005) Direct observation of the three-state folding of a single protein molecule. Science 309:2057–2060 [DOI] [PubMed] [Google Scholar]
- 18.Moffitt JR, Chemla YR, Smith SB, Bustamante C (2008) Recent advances in optical tweezers. Annu Rev Biochem 77:205–228 [DOI] [PubMed] [Google Scholar]
- 19.Greenleaf WJ, Woodside MT, Block SM (2007) High-resolution, single-molecule measurements of biomolecular motion. Annu Rev Biophys Biomol Struct 36:171–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dudko OK, Hummer G, Szabo A (2008) Theory, analysis, and interpretation of single-molecule force spectroscopy experiments. Proc Natl Acad Sci U S A 105:15755–15760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hao Y, Canavan C, Taylor SS, Maillard RA (2017) Integrated method to attach DNA handles and functionally select proteins to study folding and protein-ligand interactions with optical tweezers. Sci Rep 7(1):10843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.England JP et al. (2018) Switching of the folding-energy landscape governs the allosteric activation of protein kinase A. Proc Natl Acad Sci 115:E7478–E7485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dudko OK (2016) Decoding the mechanical fingerprints of biomolecules. Q Rev Biophys 49:e3. 10.1017/S0033583515000220 [DOI] [PubMed] [Google Scholar]
- 24.Marszalek PE, Li H, Fernandez JM (2001) Fingerprinting polysaccharides with single-molecule atomic force microscopy. Nat Bio-technol 19:258–262. 10.1038/85712 [DOI] [PubMed] [Google Scholar]
- 25.Hao Y et al. (2019) Activation of PKA via asymmetric allosteric coupling of structurally conserved cyclic nucleotide binding domains. Nat Commun 10:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dietz H, Rief M (2006) Protein structure by mechanical triangulation. Proc Natl Acad Sci U S A 103:1244–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor SS, Ilouz R, Zhang P, Kornev AP (2012) Assembly of allosteric macromolecular switches: lessons from PKA. Nat Rev Mol Cell Biol 13:646–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su Y et al. (1995) Regulatory subunit of protein kinase A: structure of deletion mutant with cAMP binding domains. Science 269:807–813 [DOI] [PubMed] [Google Scholar]
- 29.Kim C, Cheng CY, Saldanha SA, Taylor SS (2007) PKA-I holoenzyme structure reveals a mechanism for cAMP-dependent activation. Cell 130:1032–1043 [DOI] [PubMed] [Google Scholar]
- 30.Smith SB, Cui Y, Bustamante C (2003) Optical-trap force transducer that operates by direct measurement of light momentum. Methods Enzymol 55:134–162 [DOI] [PubMed] [Google Scholar]
- 31.Wu J, Jones JM, Xuong NH, Ten Eyck LF, Taylor SS (2004) Crystal structures of RIà subunit of cyclic adenosine 5′-monophosphate (cAMP)-dependent protein kinase complexed with (R p)-adenosine 3′,5′-cyclic monophosphothioate and (S p)-adenosine 3′,5′-cyclic monophosphotioate, the phosphothioate analogues of cAM. Biochemistry 43:6620–6629 [DOI] [PubMed] [Google Scholar]
- 32.Cecconi C, Shank EA, Dahlquist FW, Marqusee S, Bustamante C (2008) Protein-DNA chimeras for single molecule mechanical folding studies with the optical tweezers. Eur Biophys J 37:729–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diller TC, Madhusudan, Xuong N-H, Taylor SS (2001) Molecular basis for regulatory subunit diversity in cAMP-dependent protein kinase: crystal structure of the type II# regulatory subunit. Structure 9:73–82 [DOI] [PubMed] [Google Scholar]
- 34.Collin D et al. (2005) Verification of the Crooks fluctuation theorem and recovery of RNA folding free energies. Nature 437:231–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liphardt J, Dumont S, Smith SB, Tinoco I, Bustamante C (2002) Equilibrium information from nonequilibrium measurements in an experimental test of Jarzynski’s equality. Science 296:1832–1835 [DOI] [PubMed] [Google Scholar]
- 36.Dudko OK, Hummer G, Szabo A (2008) Theory, analysis, and interpretation of single-molecule force spectroscopy experiments. Proc Natl Acad Sci 105:15755–15760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dudko OK, Hummer G, Szabo A (2006) Intrinsic rates and activation free energies from single-molecule pulling experiments. Phys Rev Lett 96:108101. [DOI] [PubMed] [Google Scholar]


