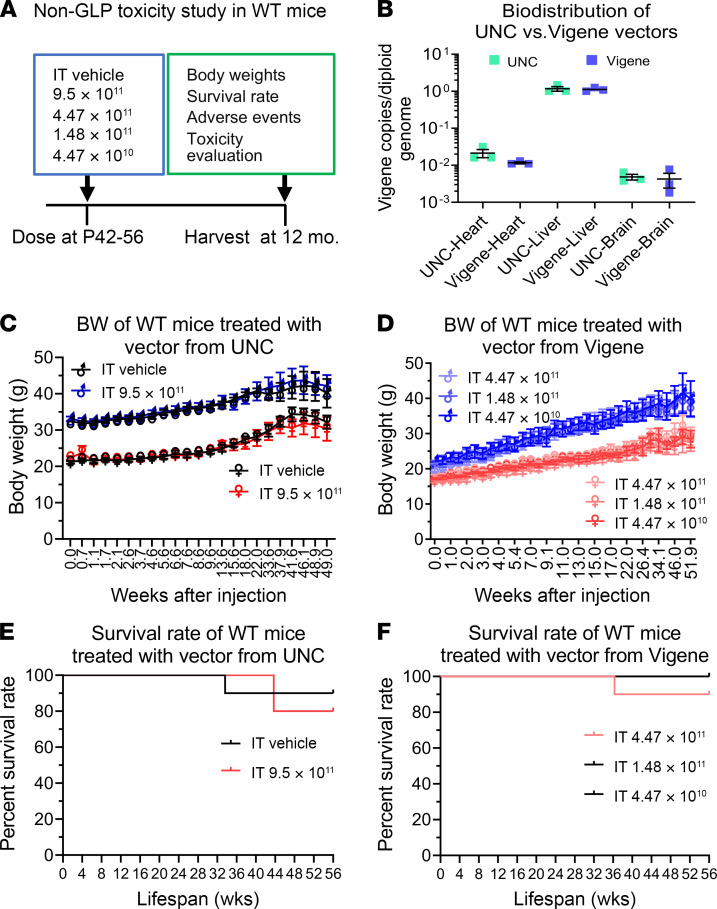
Figure 8. AAV9/MFSD8 GT does not significantly affect BW or survival rate of WT mice in non-GLP toxicity study.
(A) Experimental design of the non-GLP toxicity study in WT mice. (B) In vivo equivalence of preclinical lots of AAV9/MFSD8 vectors from UNC and Vigene. WT Mice (n = 3) in each group were injected with the vector via tail vein in a 200 μL bolus of 2 × 1011 vg/mouse. Mouse heart, liver, and brain were harvested a week later for biodistribution analysis. Data sets that passed tests for normality or homogeneity of variance were analyzed using unpaired t test with α set at 0.05. Data sets that did not pass tests for normality or homogeneity of variance were analyzed using Mann-Whitney U test with α set at 0.05. No significance was observed. (C and D) BW of WT mice (n = 5/group/sex) treated with vector from UNC (C) or Vigene (D). Two-way ANOVA with repeated measures was used for statistical analysis, and no interaction significance was observed. All data in B–D are presented as mean ± SEM. (E and F) Survival rate of WT mice (n = 5/group/sex) treated with vector from UNC (E) or Vigene (F). Data shown in Kaplan-Meier survival curve were compared with log-rank (Mantel-Cox) test. No significance was observed.