Figure 2.
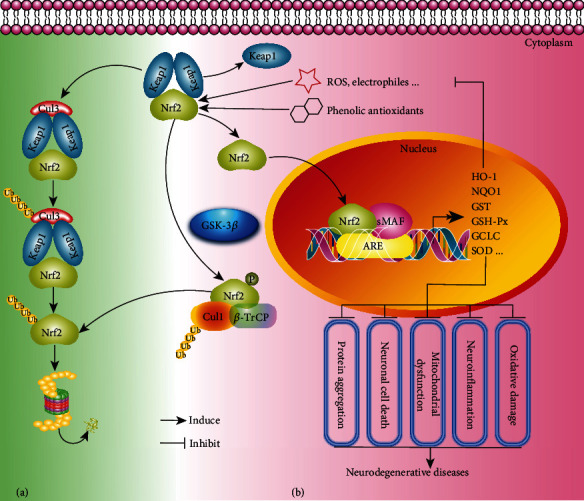
The regulation of the Keap1-Nrf2 pathway under the stimulation of ROS and electrophiles or the treatment of phenolic antioxidants in neurodegenerative diseases. Under basic conditions, Keap1, functioning as a substrate adaptor protein for Cullin3-based Cullin-RING E3 ubiquitin ligase complex around the Cullin3 (Cul3) scaffold protein, mediates the ubiquitination and proteasomal degradation of Nrf2. Under Nrf2 activation, the generated ROS or electrophiles alter the interaction between Nrf2 and its repressors under oxidative stress, resulting in the accumulation of Nrf2 in the cytoplasm and the translocation of Nrf2 into the nucleus, while the phenolic antioxidants (exogenous activator) enhance the effect of the endogenous activator on the Nrf2 pathway, thereby accelerating the dissociation of Nrf2 from Keap1 and leading to more Nrf2 translocation into the nucleus under the conditions of oxidative stress. Through the binding with Maf and ARE, Nrf2 regulates the expression of its downstream target genes, including heme oxygenase-1 (HO-1), NADPH Quinone Dehydrogenase 1 (NQO1), glutathione S-transferase (GST), glutathione peroxidase (GSH-Px), Glutamate-Cysteine Ligase Catalytic Subunit (GCLC), and superoxide dismutase (SOD). Alternatively, Nrf2 is phosphorylated by GSK-3β; then, β-transducin repeat-containing protein (β-TrCP) mediates its interaction with a Cul1 ubiquitin ligase complex to promote the proteasomal degradation of Nrf2, thereby inhibiting the expression of cytoprotective genes. The upregulation of cytoprotective genes prevents the generation of ROS levels, as well as oxidative damage, neuroinflammation, mitochondrial dysfunction, neuronal cell death, and protein aggregation.
