Figure 3.
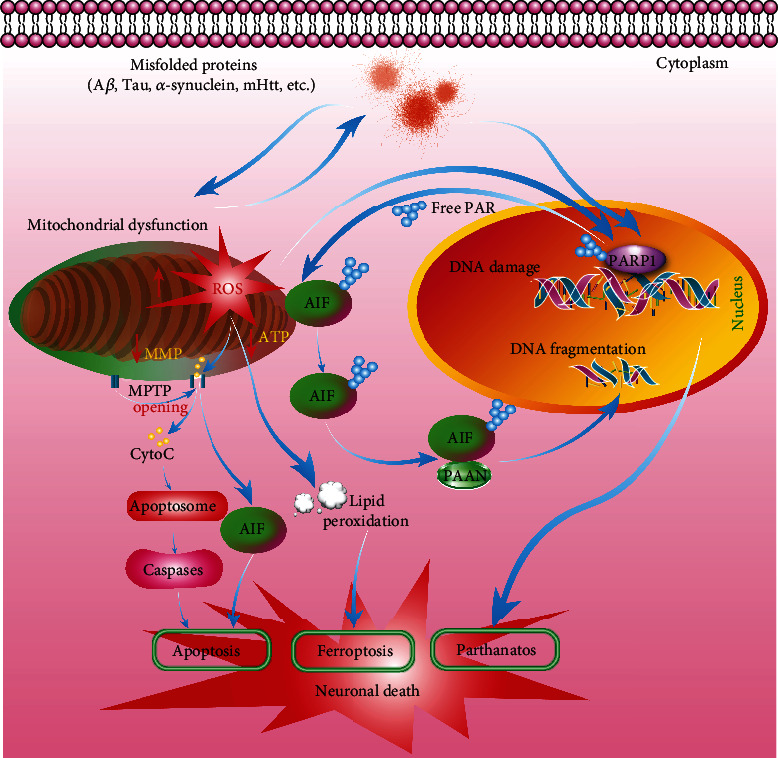
The mitochondrial dysfunction and DNA damage in neurodegenerative diseases. The increasingly accumulated misfolded proteins (Aβ, Tau, α-synuclein, mHtt, etc.) damage the normal function of mitochondria, thus resulting in the opening of the mitochondrial permeability transition pore (MPTP). The damaged mitochondria exhibit reduced ATP levels, increased ROS generation, decreased MMP, and increased release of cytochrome c (CytoC) into the cytosol, which promotes the formation of the apoptosome and subsequent proteolytical cleavage of procaspase-3 and procaspase-7, into the activated forms. Meanwhile, the loss of MMP results in the release of apoptosis-inducing factor (AIF) that is on the cytosolic side of the outer membrane of the mitochondria into the cytosol. The activation of caspases and accumulation of AIF ultimately induce neuronal cell apoptosis. In addition, the generation of large amounts of ROS induces the production and accumulation of lipid peroxidation, which indicates that neurons undergo ferroptosis. It is worth noting that the damaged mitochondria in turn further exacerbate the aggregation of misfolded proteins. In addition, the increasingly accumulated misfolded proteins induce DNA damage in the nucleus. The damaged DNA then activates PARP-1, which catalyzes PAR formation. The free PAR translocates from the nucleus to the cytosol and mitochondria where it binds AIF, inducing AIF release from the mitochondria. Then, AIF binds the parthanatos AIF-associated nuclease (PAAN) and translocates to the nucleus and causes the generation of DNA fragmentation, which induces neuronal cell death via parthanatos.
