Abstract
Background
Most solid organ transplant recipients did not develop an appreciable serologic response after 2 doses of the mRNA SARS-CoV-2 vaccine.
Methods
We analyzed the humoral response after a third dose of the BNT162b2 vaccine in 130 kidney transplant recipients, compared to 48 health care workers, and associated factors, including prevaccine cellular immune response, by evaluating intracellular cytokine production after stimulation of donor's peripheral blood mononuclear cells.
Results
After 2 doses, most of the controls (47 out of 48, 98%) and only 40% of kidney recipients (52 of 130) kidney recipients were seropositive (P < .001). Most seronegative recipients developed a serologic response after the booster (47 out 78, 60%), thus bringing the total number of seropositive recipients to 99 out of 130 (76%). After the third dose, there was a significant increase in antibodies titers in both groups. Decreased humoral response was significantly associated with an older age, lower lymphocyte count, and a lower level of antibodies before booster administration. CD4+TNFα+ and CD4+INFγ+ were correlated with mean increase in antibody titers.
Conclusions
A third dose of the BNT162b2 mRNA vaccine in kidney recipients is safe and effectively results in increased IgG anti-S levels, including in individuals who were seronegative after 2 doses. Long-term studies of the length of the immune response and protection are required.
An infection by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the resulting disease, COVID-19, have affected millions of people worldwide. Solid organ transplant (SOT) recipients are in increased risk of morbidity and mortality from COVID-19 due to their comorbidities and chronic immunosuppression state [1,2].
Vaccines to prevent SARS-CoV-2 infection are considered the most promising approach for controlling the pandemic and are being vigorously pursued. Recently, studies demonstrated that, in contrast to immunocompetent individuals, most SOT recipients did not mount an appreciable serologic response [3], [4], [5] and showed decreased cellular immunity [6] after 2 doses of the mRNA SARS-CoV-2 vaccine.
Those observations—together with a suggested correlation between breakthrough infections and lower antibody levels after 2 doses of vaccine in the general population [7] and SOT recipients [8]—have led experts to recommend administration of a booster (third) dose to certain immunocompromised individuals, including SOT recipients [9], [10], [11].
The first reports on administration of a third dose of the mRNA vaccine to SOT recipients have shown that a third dose improves the immune response without causing any short-term, serious adverse events [12,13]. However, the timing of the booster dose was less than 3 months after the second dose.
In the present study, we aimed to quantify the humoral response after the third (booster) dose of the BNT162b2 (Pfizer-BioNTech) SARS-CoV-2 mRNA vaccine, which was given 6 months after the second dose, among kidney transplant recipients, and associated factors, including magnitude of cellular immune response before the booster dose. We compared the results to a cohort of immunocompetent health care workers.
We included only participants with negative serology to SARS-CoV-2 nucleocapsid (N) protein and excluded participants with prior exposure to the virus and evaluate the immune response to the vaccine itself.
Methods and Materials
Study Design
The study group included 132 adult kidney transplant recipients who received 2 doses (21 days apart) and a third dose, at least 5 months after the second dose, of the BNT162b2 (Pfizer-BioNTech) SARS-CoV-2 mRNA vaccine.
The control group, composed of 48 immunocompetent health care workers, were vaccinated according to the same protocol as the study group.
Blood samples were collected before the booster dose was given (same day or 1 day before) and 10 to 25 days afterward. Freshly collected blood in clot activator gel tube was centrifuged at 3500 rpm for 4 minutes. The sera were separated and stored at 4°C for analysis.
The study was approved by the Tel Aviv Medical Center Institutional Ethical Review Board, and all participants provided written informed consent.
Humoral Immune Response
Levels of antibodies targeting SARS-CoV-2 spike protein (IgG S1) were measured twice using the AdviseDx SARS-CoV-2 IgG II Quant assay (Abbott, Abbott Park, IL) on an Architect i200SR analyzer (Abbott). A cutoff value ≥50AU/mL was considered a significant antibody response, as previously suggested [14]. The results of this assay have been shown to correlate with in vitro neutralization of SARS-CoV-2 [15].
Included in the study were participants who were never positive to polymerase chain reaction to SARS-CoV-2. In addition, every participant was tested to IgG antibodies against the SARS-CoV-2 nucleocapsid protein. This test was performed with an Architect i2000SR analyzer (Abbot Diagnostics, IL) and Abbott chemistry according to the manufacture instructions. A cutoff of 1.4 index (S/C) was used [15]. Individuals with detectable IgG to nucleocapsid protein were excluded.
Evaluation of Cellular Immune Response
T cell response was assessed by stimulating participant peripheral blood mononuclear cells (PBMCs) with pooled complete S-peptide mix in the presence of protein transport inhibitor, followed by staining for the activation marker (CD40L) and intracellular cytokines (TNFα and IFNγ). For this purpose, we used a SARS-CoV-2 T Cell Analysis Kits for human PBMCs (Cat# 130-128-156, Miltenyi Biotec, Germany) and performed an assay according to manufacturer instructions. Donor PBMCs were plated in a 96-well plate at a concentration of 0.5-1 × 106 PBMCs/100µL and incubated at 37°C and 5% CO2 with either pooled S-peptide mix, CytoStim for positive control, or sterile water for negative control. After 2 hours, Brefeldin A was added to each well and cells were incubated for additional 4 hours. Cells were then stained with viability dye, followed by fixation, permeabilization, and staining for surface markers (CD3, CD20, CD14, CD4, CD8, CD154) and intracellular cytokines (TNFα and IFNγ). After staining, samples were acquired using BD FACSCanto II, and 20,000 CD4+ events were collected for each sample. Analysis was performed on gated CD4+ T-cells, and the absolute number of activated INFγ+/TNFα+ double-positive cells was recorded. To evaluate the actual response rate, an activation ratio was calculated while considering the absolute number of events recorded, background activation and rate of activation relative to the positive control. For this purpose, the rate of recorded double cytokine positive CD4+ T-cells was normalized by subtracting the rate of double-positive events in the negative control sample and dividing it by the rate of the double-positive events in the CytoStim positive control, as shown in the following formula:
Immunosuppression
Induction treatment was either anti-thymocyte globulin or basiliximab, according to patient risk of rejection, in addition to methylprednisolone intravenously. Chronic immunosuppression was a combination of at least 2 medications from the following: low dose prednisone, calcineurin inhibitors, mycophenolate mofetil or mycophenolate sodium, mTOR inhibitors, or azathioprine.
Triple immunosuppression was defined as any combination of 3 different medications.
Consistent with the 2009 Kidney Disease: Improving Global Outcomes [16] guidelines for the care of kidney transplant recipients, high-dose corticosteroids were defined as methylprednisolone ≥125 mg daily or prednisone ≥40 mg daily. The attending nephrologist may have considered change of the chronic regimen according to risk for rejection, side effects, or other considerations. The change could include intensification, reduction, change of dosage, suspending, and adding or switching of a specific agent.
Clinical data were obtained from the medical charts. Data on chronic medications laboratory tests were obtained from the last clinic visit before the third vaccine dose.
Estimated glomerular filtration rate was calculated using the Modification of Diet in Renal Disease formula [17] and adjusted to body surface area (Mosteller calculation).
Statistical Analysis
Continuous variables were tested for normality using the Kolmogorov-Smirnov test and Q-Q plots and were summarized and displayed as mean (standard deviation [SD]) for normally distributed variables and as median (interquartile range [IQR]) for non-normally distributed variables and were compared by using a t test or analysis of variance if normally distributed or by Kruskal Wallis/Mann-Whitney test if non-normally distributed.
Categorical variables were displayed as number of participants and percentage. χ2 statistic was used to assess the statistical significance between groups.
Correlation between 2 continuous parameters was analyzed by Spearman analysis. To identify multicollinearity, we calculated variance inflation factors and reported the factors for values >3. Binary logistic regression models were fitted for the risk of negative serologic response including the significant variables that were found in univariate analysis.
P < .05 was considered statistically significant for all analyses. IBM SPSS Statistics for Windows, version 22, (IBM Corp., Armonk, NY) was used for all statistical analyses.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Results
Two transplant recipients were excluded from the study due to positive test of IgG antibodies to SARS-CoV-2 nucleocapsid protein as a marker of past exposure to the natural virus. Both patients had positive levels of antispike antibodies as well.
The study group consisted of 130 kidney transplant recipients who received transplants between January 1, 1996, and May 1, 2021. Four of them had liver and kidney transplants, and 8 had simultaneous kidney and pancreas transplants.
Sixteen recipients had the first and second doses of SARS-CoV2 vaccine before transplantation (and the third dose was given after transplantation).
The mean time from the transplantation was 66 months, and 25 patients (19.2%) in this group were transplanted during the year preceding the vaccination, but none of them in the 3 months before vaccination. The control group included 48 immunocompetent healthy individuals.
As shown in Table 1 , participants in the control group were younger and had a higher prevalence of women. The time intervals between the first and third dose of the vaccine, and between the third vaccine dose and the postvaccine sample collection, were similar between the 2 groups.
Table 1.
Baseline Characteristics of the Study Groups
| Parameter | Study Group | Control Group | P Value |
|---|---|---|---|
| Number | 130 | 48 | |
| Age, years | 58 (12.8) | 53.4 (11.1) | .015 |
| Sex, female (%) | 29 (60.4) | 41 (31.5) | .009 |
| BMI, kg/m2 | 27.2 (4.2) | 25.8 (4.1) | .33 |
| Time on dialysis before transplantation, months | 28 (24.6) | - | - |
| Pre-emptive transplantation, (%) | 32 (31) | - | - |
| Time since first transplantation, months | 66 (88.1) | - | - |
| First transplant (%) | 124 (95.3) | - | - |
| Etiology for kidney failure | - | - | |
| Diabetes/nephrosclerosis | 50 (38.4) | ||
| Glomerulonephritis | 31 (23.8) | ||
| Polycystic kidney disease | 29 (15.4) | ||
| Other/unknown | 29 (22.3) | ||
| Donor type, living (%) | 87 (66.9) | - | - |
| Hypertension, (%) | 94 (72.3) | - | - |
| Diabetes mellitus, (%) | 61 (46.8) | - | - |
| High dose steroids last year, (%) | 34 (26.1) | - | - |
| Anti-thymocyte globulin, (%) | 59 (45.4) | - | - |
| Anti-thymocyte globulin last year, (%) | 23 (17.7) | - | - |
| Rituximab last 12 months, (%) | 7 (5.4) | - | - |
| Low dose prednisone, (%) | 111 (85.3) | - | - |
| CNIs, (%) | 117 (90) | - | - |
| MMF, (%) | 99 (76.1) | - | - |
| Triple maintenance immunosuppression, (%) | 101 (77.6) | - | - |
| Hemoglobin, g/dL | 13.5 (1.8) | - | - |
| White blood cell count, 10e3/µL | 8.1 (2.5) | - | - |
| Neutrophils’ count, 10e3/µL | 5.3 (1.8) | - | - |
| Lymphocyte count, 10e3/µL | 1.9 (0.9) | - | - |
| Serum albumin, g/dL | 4.1 (1.9) | - | - |
| Serum creatinine, mg/dL | 1.3 (0.5) | - | - |
| eGFR, mL/min/m2 | 64.4 (20.2) | - | - |
| Period of time from 1st to 3rd dose of vaccine, Days | 206 (17.5) | 207 (11.6) | .43 |
| Period of time from 3rd dose of vaccine to serology test, days | 21 (2.1) | 20 (2.9) | .115 |
Data are expressed as mean (standard deviation) unless otherwise stated.
BMI, body mass index; CNIs, calcineurin inhibitors (tacrolimus or cyclosporin); eGFR, estimated glomerular filtration rate; MMF, mycophenolate mofetil or mycophenolate sodium.
Maintenance immunosuppression of most recipients included low-dose prednisone, tacrolimus, and mycophenolate mofetil or mycophenolate sodium.
Clinical Outcomes and Adverse Reactions After the Third Dose Administration
After receiving the third vaccine dose, 3 participants developed COVID-19. One kidney recipient was tested seronegative 21 days after the third dose and is currently admitted with severe COVID-19. Two participants—a kidney recipient and a health care worker—tested positive in the postvaccine sample and suffered only mild disease 24 and 14 days post vaccination.
In both recipients and control groups, the booster dose of vaccination was safe, with no biopsy-proven acute rejections (in the study group), severe allergic reactions, or new neurologic diagnoses (Guillain-Barre syndrome, Bell's palsy, zoster, or other neuropathies) during a mean follow-up period of 46 days (±11) after administration of the booster.
Humoral Response After a Third Vaccine Dose
After 2 doses and just before the third dose, most of the controls had detectable levels of IgG anti spike antibodies (47 out of 48, 99%), whereas only 40% of recipients (52 of 130) were seropositive (P < .001).
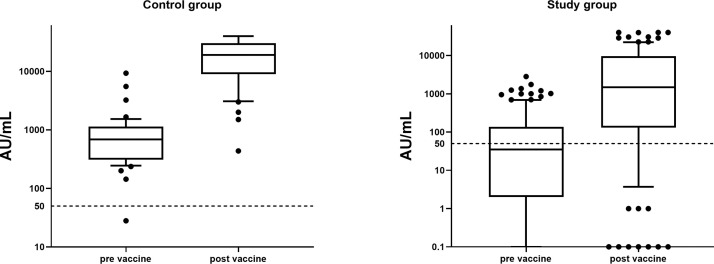
As in our previous work [4], the levels of anti-spike IgG in the recipients group (median = 35 AU/mL [IQR 0-135]) was significantly lower before the booster than in the controls (median, 687 AU/mL [IQR 308-1139]; P = .003) (Fig 1 ). However, when considering only seropositive participants, mean antibody levels were similar in both groups (median, 189 AU/mL [IQR 93-682] vs 709 AU/mL [IQR 336-1142], study vs controls, respectively; P = .43).
Fig 1.
Box plot of IgG anti S in participants before and after booster administration in study group and control. There was a significant difference before and after the booster, as well as between the groups (P < .001 for all comparisons). Dashed line indicates IgG = 50 AU/mL as cut off for seropositive level.
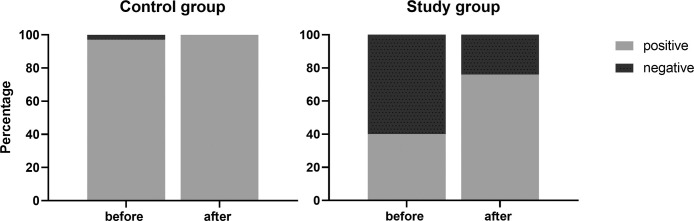
All seropositive patients in the control group and in the study group remained seropositive after the third dose of the vaccine. In the control group, the only seronegative individual converted after receiving the third vaccine dose. Among the study group, most of the 78 kidney recipients who were seronegative before the booster developed a serologic response of significant levels of IgG anti S after the booster (47 of 78, 60%) (Fig 2 ), thus bringing the number of seropositive recipients to 99 of 130 (76%, as compared to just 40% after 2 vaccine doses).
Fig 2.
Negative and positive humoral response, before and after the booster administration, in kidney recipients and controls (P < .001).
After the third dose, there was a significant increase in antibodies titers in both groups. The mean increase in antibody titers was significantly higher in the controls (median, 1278 AU/mL [IQR 68-7075] vs 28,358 AU/mL [IQR 15,951-36,766] for study vs controls, respectively; P < .001) (Fig 2).
Factors Related to Humoral Immune Response
Univariate and multivariate analysis of variables associated with the risk of negative humoral response after the third vaccine dose are demonstrated in Table 2 .
Table 2.
Univariate and Multivariate Analysis of Variables Related to Risk of Negative Response After the Third Dose in Kidney Transplant Recipients
| Univariate Analysis |
Multivariate Analysis |
|||||
|---|---|---|---|---|---|---|
| Variable | Exp (B) | 95% CI | P value | Exp (B) | 95% CI | P value |
| Age (y) | 1.07 | 1.02-1.12 | .002 | 1.054 | 1.01-1.14 | .03 |
| Antibody level before booster (AU/mL) | 0.93 | 0.90-0.97 | <.001 | 0.93 | 0.89-0.98 | .002 |
| Sex | 0.95 | 0.4-1.7 | .92 | - | - | - |
| Donor (deceased vs living) | 1.3 | 0.79-3.3 | .52 | - | - | - |
| eGFR, mL/min | 0.97 | 0.95-0.99 | .03 | 0.97 | 0.92-1.01 | .15 |
| BMI | 0.97 | 0.83-1.15 | .62 | - | - | - |
| Time on dialysis (mo) | 1.1 | 0.9-1.27 | .12 | - | - | - |
| MMF | 1.04 | 1.01-1.3 | .05 | 1.01 | 0.99-1.3 | .28 |
| Lymphocyte count | 0.81 | 0.73-0.75 | .03 | 0.97 | 0.91-0.99 | .04 |
| Time since transplantation, months | 0.97 | 0.92-1.06 | .07 | 0.97 | 0.96-1.11 | .17 |
BMI, body mass index; CI, confidence interval; eGFR, estimated glomerular filtration rate; MMF, mycophenolate mofetil or mycophenolate sodium.
Age was inversely correlated to mean increase in antibody titers in both groups (correlation coefficient, –0.359 [P < .001] and –0.213 [P = .04] for study and controls, respectively). In addition, levels of antibody before the third dose were significantly correlated with increased titer after it (correlation coefficient, 0.515 and 0.64, for study and control groups, respectively; P < .001, for both).
Decreased humoral response was significantly correlated with a lower lymphocyte count, and a lower level of antibodies before the vaccine booster administration. In addition, every year of age increased the risk of having a negative serology by 5%.
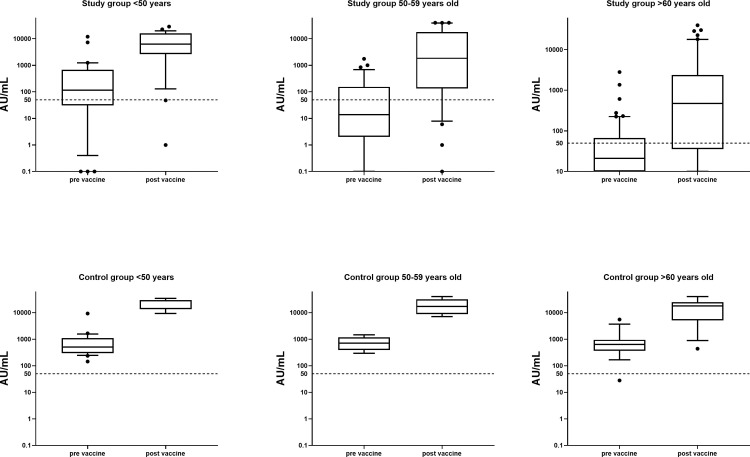
The differences in the magnitude of the humoral immune response to the booster in both groups according to patient age are shown in Figure 3 . Comparison of the participants (controls and kidney transplant recipients) in the different age groups (<50, 50-59, and >60) has revealed significant benefit for the younger groups in the magnitude of antibody level after the booster administration and in the delta of antibody levels before and after the booster. The difference in mean antibody levels before booster administration between participants of the control group aged <50 and 50 to 59 years did not reached a statistical difference (P = .06); older individuals (>60 years old) in the control group had a significantly lower antibody level.
Fig 3.
IgG anti S levels in study cohorts subtracted to age groups of below 50 years, 50 to 59 years, and above 60 years. Dashed line indicates IgG = 50 AU/mL as cut off for seropositive level. *P = 0.06 for controls age <50 years and 50 to 59 years before booster administration. For all other comparisons of study vs control, before vs after booster: P < .05.
For all age groups, serologic response was lower in study verus control group both before and after receiving the booster. However, booster vaccination lead to significant increase of antibody levels in both groups and all age groups (P < .05 for all comparisons).
Cellular Immune Response
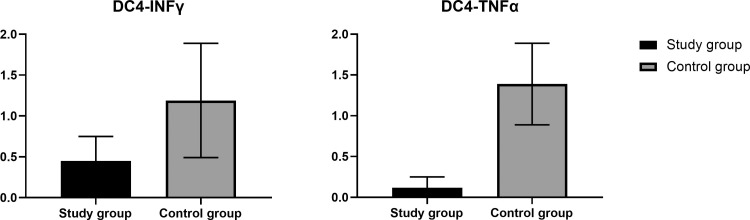
Intracellular cytokine staining stimulated PBMCs was performed before the third vaccine dose in 14 healthy controls and 14 kidney transplant recipients. For this purpose, donors’ cells were stimulated with a pooled S-peptide mix or controls, and intracellular cytokine production was evaluated by flow cytometry, gating on the CD4+ population. Mean percentage of CD4+TNFa+ and CD4+INFγ+ cells was significantly higher in controls compare to kidney recipients (Fig 4 ). In a correlation analysis, CD4+TNFa+ significantly correlated with the mean increase in antibody titers after the vaccine boost (correlation coefficient, 0.58; P = .029), and correlation of CD4+INFγ+ with mean antibody increase showed a trend toward statistical significance (correlation coefficient, 0.49; P = .07). Healthy controls and patients who underwent transplants did not show appreciable CD8 response (data not shown).
Fig 4.
Mean and standard deviation of CD4+INFγ+ and CD4+TNFα+ (activation ratio) in participants of both study group. P = .048 and .046, respectively
Discussion
Data evaluating the response to SARS-CoV-2 vaccines have been exponentially accumulating over the last several months. There is a consistent evidence that both humoral and cellular immune responses to SARS-CoV-2 vaccines are significantly reduced in kidney transplant recipients [18,19].
Observation of the waning immunity—demonstrated by decreased antibody levels in vaccinated subjects as well as correlation between breakthrough infections and the time that has passed since the second vaccine dose—has led experts, including those in the U.S. Food and Drug Administration, to recommend giving a booster vaccine dose to individuals over the age of 65, as well as immunocompromised patients and health care workers of any age, for whom the time interval since their second vaccine dose is over 5 months [20,21].
This study describes the anti-S1 IgG antibody response of kidney transplant recipients after a booster dose of the BNT162b2 (Pfizer-BioNTech) vaccine, and highlights parameters associated with it. We also studied the cellular response after the second dose of vaccine and showed it could serve as prediction for post-booster humoral response.
Our pivotal finding was that most kidney recipients (60%) remained seronegative after the second dose of the vaccine, as compared to ∼3% in the healthy controls. However, most kidney recipients who did not develop response after the second dose showed a seroconversion after the booster vaccine administration, raising the level of seropositivity in the of recipients to 76%.
The clinical importance of this laboratory finding is supported by growing evidences, suggesting that elapsed time has a major role in breakthrough infections due to wanning immunity and declining of antibody titers [20]. Bergwerk et al [7] described breakthrough infections with SARS-CoV-2 in a cohort of health care workers and a correlation of these infections with the declining of neutralizing antibody titers in the peri-infection period.
According to the data of recent studies, including data from Israel, the booster is effective in reducing infections, as well as the rates of severe infections and hospitalizations due to COVID-19 [22,23].
The data on the cellular immune response performed in our study demonstrated, unsurprisingly, significantly reduced cellular response in kidney transplant recipients in comparison to healthy controls. Somehow encouraging is our finding, showing that the presence of measurable levels of cellular immunity was associated with a better response following the third vaccine dose, despite lower absolute antibody levels.
The predictors of blunt humoral response among transplant recipients after third dose of vaccine were decreased antibody response after the second dose, advanced age, and low lymphocyte count at the time of booster administration.
Advanced age was consistently related to reduced antibody response in immunocompetent patients after COVID-19 [24], as well as after SARS-CoV-2 vaccination [25,26], and in kidney transplant recipients [27]. In concordance with previous studies, our study shows inferior serologic response after the third dose of vaccine, which is similar to the 2-dose vaccination schedule, in addition to a lower surge in net antibody titers after the booster in the elderly patients.
Recent studies demonstrated that the serologic response to vaccination against SARS-CoV-2 was affected by the net burden of patient immunosuppression [4,5]. After adjusting to other variables, we were not able to show this correlation in the present study. Although it is tempting to speculate that booster vaccinations overcome the barrier of high burden of immunosuppression, a more realistic explanation might be that the lower titer of antibody before the booster has a strong statistical significancy, which overcome other clinical parameters related to it.
Strengths of this study include its novelty. This is the first published data on booster administration 6 months after the second dose of the vaccine and correlation to cellular response before the third dose. Validation of the findings were done by a comparison to immunocompetent individuals who were vaccinated on similar schedule. Exclusion of participants with IgG antibodies to nucleocapsid protein eliminates the possibility of response to the virus itself and therefore contributes to the validation of our results.
Limitations of the study include a short follow-up period and absence of assessing the cellular immune response after the third dose, thus preclude us to address full spectrum of its immunogenicity as well as the clinical implications.
Despite these limitations, the accumulating data of significantly reduced immune response to SARS-CoV-2 vaccines in transplant recipients warrant prompt consideration and further studies about possible ways to improve immunogenicity in this vulnerable population, as well as the precise ideal timing of vaccination doses.
Conclusions
A third dose of the Pfizer- BioNTech BNT162b2 vaccine in kidney transplant recipients is safe and effectively results in increased IgG anti-S levels, including in transplant recipients who were seronegative after 2 doses. Our results strongly support the current recommendations of administration of booster mRNA vaccine to solid organ transplant recipients. However, long-term studies of the length of the immune response and protection are required.
Acknowledgments
Serum samples were collected from patients with help from the TLV Biobank and MIDGAM-Israel National Biobank for Research. This study was performed in collaboration with the Israeli Ministry of Health.
References
- 1.Caillard S, Anglicheau D, Matignon M, Durrbach A, Greze C, Frimat L, et al. An initial report from the French SOT COVID Registry suggests high mortality due to COVID-19 in recipients of kidney transplants. Kidney Int. 2020;98:1549–1558. doi: 10.1016/j.kint.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caillard S, Chavarot N, Francois H, Matignon M, Greze C, Kamar N, et al. Is COVID-19 infection more severe in kidney transplant recipients? Am J Transplant. 2021;21:1295–1303. doi: 10.1111/ajt.16424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyarsky BJ, Werbel WA, Avery RK, Tobian AAR, Massie AB, Segev DL, et al. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA. 2021;325:1784–1786. doi: 10.1001/jama.2021.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grupper A, Rabinowich L, Schwartz D, Schwartz IF, Ben-Yehoyada M, Shashar M, et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant. 2021;21:2719–2726. doi: 10.1111/ajt.16615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyarsky BJ, Werbel WA, Avery RK, Tobian AAR, Massie AB, Segev DL, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204–2206. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stumpf J, Siepmann T, Lindner T, Karger C, Schwöbel J, Anders L, et al. Humoral and cellular immunity to SARS-CoV-2 vaccination in renal transplant versus dialysis patients: a prospective, multicenter observational study using mRNA-1273 or BNT162b2 mRNA vaccine. Lancet Reg Health Eur. 2021;23 doi: 10.1016/j.lanepe.2021.100178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergwerk M, Gonen T, Lustig Y, Amit S, Lipsitch M, Cohen C, et al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385:1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anjan S, Natori Y, Fernandez Betances AA, Agritelley MS, Mattiazzi A, Arosemena L, et al. Breakthrough COVID-19 infections after mRNA vaccination in solid organ transplant recipients in Miami, Florida. Transplantation. 2021;105:e139–e141. doi: 10.1097/TP.0000000000003902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Evidence to recommendation framework: an additional dose of mRNA COVID-19 vaccine following a primary series in immunocompromised people. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-08-13/02-COVID-Dooling-508.pdf. Accessed August 14, 2021.
- 10.Centers for Disease Control and Prevention. Data and clinical considerations for additional doses in immunocompromised people. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-07/07-COVID-Oliver-508.pdf. Accessed July 23, 2021.
- 11.Emergency use authorization (EUA) of the Pfizer-BioNTech COVID-19 vaccine to prevent coronavirus. Fact sheet for healthcare providers administering vaccine. Available at: https://www.fda.gov/media/144413/download. Accessed August 23, 2021.
- 12.Benotmane I, Gautier G, Perrin P, Olagne J, Cognard N, Fafi-Kremer S, et al. Antibody response after a third dose of the mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients with minimal serologic response to 2 doses. JAMA. 2021;326:1063–1065. doi: 10.1001/jama.2021.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Werbel WA, Boyarsky BJ, Ou MT, Massie AB, Tobian AAR, Garonzik-Wang JM, et al. Safety and immunogenicity of a third dose of SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Ann Intern Med. 2021;174:1330–1332. doi: 10.7326/L21-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abbott Core Laboratory. SARS-CoV-2 immunoassay. Available at: https://www.corelaboratory.abbott/int/en/offerings/segments/infectious-disease/sars-cov-2. Accessed April 1, 2021.
- 15.Perkmann T, Perkmann-Nagele N, Breyer MK, Breyer-Kohansal R, Burghuber OC, Hartl S, et al. Side-by-side comparison of three fully automated SARS-CoV-2 antibody assays with a focus on specificity. Clin Chem. 2020;66:1405–1413. doi: 10.1093/clinchem/hvaa198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Disease Kidney. Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9(Suppl 3):S1–155. doi: 10.1111/j.1600-6143.2009.02834.x. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 18.Sattler A, Schrezenmeier E, Weber UA, Potekhin A, Bachmann F, Straub-Hohenbleicher H, et al. Impaired humoral and cellular immunity after SARS-CoV2 BNT162b2 (Tozinameran) prime-boost vaccination in kidney transplant recipients. J Clin Invest. 2021;131 doi: 10.1172/JCI150175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertrand D, Hamzaoui M, Lemée V, Lamulle J, Hanoy M, Laurent C, et al. Antibody and T cell response to SARS-CoV-2 messenger RNA BNT162b2 vaccine in kidney transplant recipients and hemodialysis patients. J Am Soc Nephrol. 2021;32:2147–2152. doi: 10.1681/ASN.2021040480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldberg Y, Mandel M, Bar-On YM, Bodenheimer O, Freedman L, Haas EJ, et al. Waning immunity of the BNT162b2 vaccine: a nationwide study from Israel. medRxiv. 2021.08.24.21262423, 2021. Available at: https://www.medrxiv.org/content/10.1101/2021.08.24.21262423v1. Accessed September 2, 2021.
- 21.U.S. Food and Drug Administration. Joint statement from HHS public health and medical experts on COVID-19 booster shots. Available at:https://www.fda.gov/news-events/press-announcements/joint-statement-hhs-public-health-and-medical-experts-covid-19-booster-shots. Accessed September 22, 2021.
- 22.Israel A, Shenhar Y, Green I, Merzon E, Golan-Cohen A, Schäffer AA, et al. Large-scale study of antibody titer decay following BNT162b2 mRNA vaccine or SARS-CoV-2 infection. medRxiv. Available at: https://www.medrxiv.org/content/10.1101/2021.08.19.21262111v1. Accessed September 4, 2021. [DOI] [PMC free article] [PubMed]
- 23.Bar-On YM, Goldberg Y, Mandel M, Bodenheimer O, Freedman L, Kalkstein N, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385:1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klein SL, Pekosz A, Park HS, Ursin RL, Shapiro JR. Sex, age, and hospitalization drive antibody responses in a COVID-19 convalescent plasma donor population. J Clin Invest. 2020;130:6141–6150. doi: 10.1172/JCI142004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jimenez M, Campillo NE, Canelles M. COVID-19 vaccine race: analysis of age-dependent immune responses against SARS-CoV-2 indicates that more than just one strategy may be needed. Curr Med Chem. 2021;28:3964–3979. doi: 10.2174/0929867327666201027153123. [DOI] [PubMed] [Google Scholar]
- 26.Anderson EJ, Rouphael NG, Widge AT, Jackson LA, Roberts PC, Makhene M, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383:2427–2438. doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grupper A, Katchman E, Ben-Yehoyada M, Rabinowich L, Schwartz D, Schwartz IF, et al. Kidney transplant recipients vaccinated before transplantation maintain superior humoral response to SARS-CoV-2 vaccine. Clin Transplant. 2021;35:e14478. doi: 10.1111/ctr.14478. [DOI] [PMC free article] [PubMed] [Google Scholar]