Abstract
Background
The urinary levels of volatile organic compound (VOC) metabolites provide individual exposure levels compared to data obtained by measuring these compounds in ambient air. We aimed to investigate the association between personal urinary concentrations of VOC metabolites and symptoms of atopic dermatitis in schoolchildren.
Methods
Nine urinary VOC metabolites were analyzed from urine samples of 149 children. Diagnosis of atopic dermatitis was determined using standardized questionnaires. Pediatricians visited the schools and rated the severity of symptoms using the SCORing Atopic Dermatitis (SCORAD) in all children.
Results
Forty-five children (30.2%) had atopic dermatitis based on the International Study of Asthma and Allergies in Childhood (ISAAC) results and 35 children (23.8%) had symptoms of atopic dermatitis with positive SCORAD index values (defined as SCORAD ≥ 5). Children with benzylmercapturic acid detected in personal urines were associated with presence of atopic dermatitis and positive SCORAD index values. Children in the highest quartile of mandelic acid concentration were associated with presence of atopic dermatitis and positive SCORAD results.
Conclusion
Personal exposure to VOCs, as indicated by urinary levels of VOC metabolites, was associated with presence of atopic dermatitis and the SCORAD index value.
Keywords: Volatile Organic Compounds, Urinary Metabolite, Atopic Dermatitis
Graphical Abstract
INTRODUCTION
Anthropogenic volatile organic compounds (VOCs), such as formaldehyde, benzene, and xylene, are major indoor air pollutants produced that evaporate from various sources at room temperature.1 VOCs can cause irritation and allergic inflammation,2 and there is a plausible link of exposure to VOCs with the onset and exacerbation of atopic dermatitis.1 Atopic dermatitis is a chronic inflammatory skin disease that has become a global health problem because of its increasing incidence during recent decades.3 Research indicates that air pollutants have contributed to the increasing incidence of atopic dermatitis.4
However, previous studies that examined the effects of VOCs on atopic dermatitis had limitations,5,6,7,8,9,10,11 so this relationship remains inconclusive. In particular, these studies mainly employed air-sampling methods to estimate exposure to VOCs, therefore the effects of exposure to different environments and differences in exposure at the individual level were not under consideration. Several studies suggested possible adverse effects of exposure to indoor air pollutants and found that exposure increased the incidence or exacerbated atopic dermatitis,5,6,7 but other studies reported no direct link.8,9,10 Authors of a systematic review concluded that more qualified and evidence-based examinations are necessary to establish a definite relationship between VOC exposure and allergic diseases.11
We monitored biomarkers of VOC exposure—urinary concentrations of VOC metabolites—a widely accepted method used to quantify exposure because it accounts for differences in route of exposure, residential environment, and personal lifestyle.2 There are virtually no population-level data on the burden of indoor contaminants based on measurements of urinary concentrations of VOC metabolites to childhood atopic dermatitis. We focused on the associations between atopic dermatitis and skin inflammations represented as SCORing Atopic Dermatitis (SCORAD) index with the urinary concentrations of 9 representative metabolites of VOCs in 7-year-old school children.
METHODS
Children from 11 elementary schools were enrolled and examined between June and July, 2016. All children were in the first grade (7–8 years old). The 408 children who agreed to physical examinations and properly completed the structured questionnaire were considered eligible. Demographic characteristics (age, sex, year of birth, height, weight), details regarding allergy, environment, birth history, and parental history were obtained using questionnaires. Body mass index (BMI) was calculated to derive BMI z-score. Among the urine provided by these participants, samples with inadequate volume or quality were excluded and 149 samples were finally prepared for metabolomics assays.
Definition of main outcomes
Children with an affirmative response of the following questions of the International Study of Asthma and Allergies in Childhood (ISAAC) were defined as atopic dermatitis: “Has your child ever had an itchy rash which was coming and going for at least six months?”. Diagnosis of asthma and allergic rhinitis were also determined by characteristic symptoms during the previous 12 months, based on the results of the ISAAC questionnaire.
Physicians who visited the schools assessed the SCORAD index of each participant by skin examinations.12,13,14 At the time of skin examinations, physicians were not aware to the questionnaire answers. We considered subjects with SCORAD < 5 as “negative,” and those with SCORAD ≥ 5 as “positive.”15 In addition, we divided the results of SCORAD into three classes based on index values: mild (< 25), moderate (25–50), and severe (> 50).12
Measurements of urinary VOC metabolites
Personal exposures to VOCs were assessed using urine samples, a non-invasive procedure with minimal burden for children, which were collected on the same day as the skin examinations. Two of the 9 VOC metabolites (m-methyl-benzoic acid and phenylmercapturic acid) were below the limit of detection (LoD) in most samples and were not used for analyses. The 7 quantifiable urinary VOC metabolites (parent compounds) were: tt-cc-muconic acid (benzene), phenylglyoxylic acid (styrene), o-methylbenzoic acid (xylene), p-methylbenzoic acid (xylene), mandelic acid (benzene), benzylmercapturic acid (toluene), and thiazolidine-4-carboxylic acid (TZCA, formaldehyde). A simple high performance liquid chromatography method was developed for measurement of tt-cc-muconic acid, benzylmercapturic acid, aromatic carboxylic acids (phenylglyoxylic acid, o-/p-/m-methylbenzoic acid, and mandelic acid), and benzylmercapturic acid. A commercial Sep-Pak silica gel cartridge (Waters; Millipore Corp., Burlington, MA, USA) was used to determine TZCA. All mass spectra were obtained using an Agilent 6890/5973 N instrument for analyte identification. Calibration curves were established using derivatization and extraction, and the peak area of each internal standard was used for quantification.16 The urinary concentration of each VOC metabolite was expressed relative to grams of urinary creatinine (g Cr). For analysis of the relationships between VOC concentrations and atopic dermatitis, subjects were divided into two groups (high VOC and low VOC) for each compound based on the median concentration (Supplementary Table 1). Benzylmercapturic acid (LoD: 0.25 μg/g Cr) was detectable in only 29 children and tt-cc-muconic acid (LoD: 14 μg/g Cr) was only detectable in 55 children, and so subjects were divided into non-detected and detected groups for these compounds. When the association was insignificant based on analysis of low and high concentration groups, subjects were divided into quartiles for further analysis.
Specific IgE test
Skin prick tests were performed for 22 common allergens, 13 aeroallergens (house dust mites [Dermatophagoides farina, Dermatophagoides pteronyssinus], cat dander, dog dander, cockroach, birch, oak, elm, orchard grass, Japanese hop, mugwort, ragweed, and Alternaria) and 9 food allergens (cow’s milk, egg white, peanut, wheat, walnut, shrimp, apple, kiwi, celery). A mean wheal diameter greater than 3 mm was considered positive. Children with positive results to at least one allergen were considered to have allergen sensitization.
Statistics
The χ2 test was used to compare categorical variables. A generalized linear regression with a logit function that adjusted for confounding factors (sex, BMI z-score, prematurity and/or low birth weight, aeroallergen sensitization, current asthma, and current allergic rhinitis) was used to determine the associations. Associations between urinary VOC levels with the presence of atopic dermatitis, and with positive SCORAD results were analyzed based on adjusted odds ratio (aOR) and 95% confidence interval (CI). Non-parametric tests were used where appropriate. A P value below 0.05 was considered significant. Statistical analyses were performed using SPSS version 23.0 (IBM Co, Armonk, NY, USA). The strength of the effect of each urinary VOC metabolite on atopic dermatitis was ranked using the nonparametric random forest method with the package for R system version 2.8.2 (R Foundation, Vienna, Austria).
Ethics statement
The research protocol was approved by the Institutional Review Board of CHA University (2016-04-031). Written informed consent was obtained from the parents or caregivers of all study children.
RESULTS
A total of 149 children met the study criteria, completed the physical examinations, and provided urine samples (Table 1). Eighty-eight children were boys (59.1%) and the mean age was 7.1 years. Based on the ISAAC questionnaire, the prevalence of atopic dermatitis was 30.2% (n = 45). Based on the SCORADs, 17 children (11.6%) had mild symptoms and 18 (12.2%) had moderate to severe symptoms. Seventy-two children (48.3%) were exposed to second-hand smoke, 18 (13.1%) were premature, and 8 (5.4%) had low birth weight. The skin tests (performed in 147 children) indicated that 64 children (43.5%) were sensitized to at least 1 allergen and 83 (56%) were not sensitized to any tested allergen.
Table 1. Demographic and clinical characteristics of enrolled children (N = 149)a .
| Characteristics | Values | |
|---|---|---|
| Demographics | ||
| Age, yr | 7.11 (7.05–7.17) | |
| Sex, male | 88 (59.1) | |
| Anthropometrics | ||
| BMI z-score | 0.02 (−0.15–0.19) | |
| Height, cm | 121.7 (120.9–122.6) | |
| Allergic diseaseb | ||
| Asthma | 14 (9.5) | |
| AR | 76 (51.0) | |
| Atopic dermatitis | 45 (30.2) | |
| Risk factors | ||
| Passive smoke exposure | 72 (48.3) | |
| Prematurity | 18 (13.1) | |
| Low birth weight | 8 (5.4) | |
| Parental allergic history | ||
| Parental asthma | 12 (9.2) | |
| Parental AR | 59 (41.5) | |
| Parental atopic dermatitis | 23 (15.4) | |
| Allergen sensitizationc | ||
| Non-sensitization | 83 (56.5) | |
| Mono-sensitization | 40 (27.2) | |
| Poly-sensitization | 24 (16.3) | |
| SCORAD index | ||
| Controls (0) | 112 (76.2) | |
| Mild (< 25) | 17 (11.6) | |
| Moderate to severe (25–50) | 18 (12.2) | |
Values are presented as mean (95% confidence interval), number (%), or median (interquartile range).
BMI = body mass index, AR = allergic rhinitis, SCORAD = SCORing Atopic Dermatitis.
aData were missing for asthma (n = 10), prematurity (n = 12), parental asthma (n = 18), parental AR (n = 7), allergic sensitization (n = 2), and SCORAD result (n = 2).
bCurrent allergic history (12 months) based on the International Study of Asthma and Allergies in Childhood (ISSAC).
cAllergy to 13 aeroallergens and 9 food allergens, as described in the Methods.
Relationship of atopic dermatitis and VOC concentrations
We first compared controls and children with atopic dermatitis based on ISAAC results (Table 2). The results indicated that benzylmercapturic acid was more common in children with atopic dermatitis (19.5% vs. 13.5%, P = 0.005), consistent with the results of the multivariate analysis for this compound (adjusted odds ratio [aOR], 3.52; 95% confidence interval [CI], 1.34–8.94; P = 0.008). Comparison of the first and fourth quartiles for mandelic acid indicated that atopic dermatitis was also associated with mandelic acid concentration (aOR, 3.77; 95% CI, 1.19–11.92; P = 0.024).
Table 2. Multivariate analysis of the association of VOC metabolites with AD based on ISAAC results (N = 149).
| VOC metabolite | AD | SCORAD indexa | ||||
|---|---|---|---|---|---|---|
| Control | Subjects | P value | aOR (95% CI) | P value | ||
| Benzylmercapturic acid | ||||||
| ND | 90 (86.5) | 30 (66.7) | Ref. | |||
| Detected | 14 (13.5) | 15 (33.3) | 0.005 | 3.52 (1.34–8.94) | 0.008 | |
| p-methyl-benzoic acid | ||||||
| Low | 55 (52.9) | 19 (42.2) | Ref. | |||
| High | 49 (47.1) | 26 (57.8) | 0.232 | 1.64 (0.79–3.41) | 0.182 | |
| o-methyl-benzoic acid | ||||||
| Low | 49 (47.1) | 25 (55.6) | Ref. | |||
| High | 55 (52.9) | 20 (44.4) | 0.344 | 0.72 (0.35–1.51) | 0 | |
| tt-cc-muconic acid | ||||||
| ND | 68 (65.4) | 26 (57.8) | Ref. | |||
| Detected | 36 (34.6) | 19 (42.2) | 0.377 | 1.52 (0.72–3.21) | 0.273 | |
| Phenylglyoxylic acid | ||||||
| Low | 52 (50.0) | 22 (48.9) | Ref. | |||
| High | 52 (50.0) | 23 (51.1) | 0.901 | 1.29 (0.62–2.67) | 0.502 | |
| Thiazolidine-4-carboxylic acid | ||||||
| Low | 50 (48.1) | 24 (53.3) | Ref. | |||
| High | 54 (51.9) | 21 (46.7) | 0.556 | 0.78 (0.38–1.62) | 0.502 | |
| Mandelic acidb | ||||||
| Q1 | 30 (28.8) | 7 (15.6) | Ref. | |||
| Q2 | 27 (26.0) | 10 (22.2) | 1.82 (0.54–6.12) | 0.335 | ||
| Q3 | 26 (25.0) | 12 (26.7) | 1.67 (0.53–5.26) | 0.384 | ||
| Q4 | 21 (20.2) | 16 (35.6) | 0.022 c | 3.77 (1.19–11.92) | 0.024 | |
Values are presented as number (%). Bold values are statistically significant.
AD = atopic dermatitis, ND = not detected, SCORAD = SCORing Atopic Dermatitis, VOC = volatile organic compound, ISAAC = International Study of Asthma and Allergies in Childhood, aOR = adjusted odds ratio, CI = confidence interval.
aAdjusted for sex, BMI z-score, prematurity or/and low birth weight, allergen sensitization (no vs. yes), secondary smoking, current asthma, and current allergic rhinitis using a generalized linear regression with the logit function.
bOnly mandelic acid has shown significant linear relationships when range of concentration divided into four groups with equal intervals, therefore presented into quantiles.
c P value for a linear relationship.
Relationship of SCORAD results and VOC concentrations
We used the same analysis to compare controls with children who had positive SCORAD results (Table 3). The results indicated that benzylmercapturic acid was more common in children with positive SCORAD results (34.3% vs. 15.2%; P = 0.007), consistent with the results of the multivariate analysis for this compound (aOR, 3.62; 95% CI, 1.41–9.29; P = 0.007). The results were similar for p-methylbenzoic acid (46.4% vs. 65.7%, P = 0.046; aOR, 2.47; 95% CI, 1.09–5.61; P = 0.03) and mandelic acid (18.8% vs. 45.7%, P = 0.015; aOR, 3.92; 95% CI, 1.30–12.00; P = 0.017). The association was similar when analysis was performed using SCORAD results stratified as mild, moderate, and severe (data not shown) or when considered as a continuous variable (Supplementary Table 2).
Table 3. Multivariate analysis of the association of VOC metabolites with atopic dermatitis based on SCORAD results (N = 147)a .
| Variables | SCORAD indexa | |||||
|---|---|---|---|---|---|---|
| Negative | Positive | P value | aOR (95% CI) | P value | ||
| Benzylmercapturic acid | ||||||
| LoD | 95 (84.8) | 23 (65.7) | Ref. | |||
| Detected | 17 (15.2) | 12 (34.3) | 0.013 | 3.62 (1.41–9.29) | 0.007 | |
| p-Methyl-benzoic acid | ||||||
| Lowb | 60 (53.6) | 12 (34.3) | Ref. | |||
| High | 52 (46.4) | 23 (65.7) | 0.046 | 2.47 (1.09–5.61) | 0.030 | |
| o-Methyl-benzoic acid | ||||||
| Lowb | 54 (48.2) | 19 (54.3) | Ref. | |||
| High | 58 (51.8) | 16 (45.7) | 0.531 | 0.68 (0.30–1.51) | 0.339 | |
| tt-cc-Muconic acid | ||||||
| LoD | 70 (62.5) | 22 (62.9) | Ref. | |||
| Detected | 42 (37.5) | 13 (37.1) | 0.970 | 0.94 (0.42–2.10) | 0.882 | |
| Phenylglyoxylic acid | ||||||
| Lowb | 54 (48.2) | 19 (54.3) | Ref. | |||
| High | 58 (51.8) | 16 (45.7) | 0.531 | 0.76 (0.35–1.68) | 0.501 | |
| Thiazolidine-4-carboxylic acid | ||||||
| Lowb | 55 (49.1) | 19 (54.3) | Ref. | |||
| High | 57 (50.9) | 16 (45.7) | 0.593 | 0.76 (0.34–1.66) | 0.485 | |
| Mandelic acid | ||||||
| Q1 | 29 (25.9) | 7 (20.0) | Ref. | |||
| Q2 | 30 (26.8) | 6 (17.1) | 1.10 (0.30–4.02) | 0.881 | ||
| Q3 | 32 (28.6) | 6 (17.1) | 0.87 (0.25–3.01) | 0.775 | ||
| Q4 | 21 (18.8) | 16 (45.7) | 0.015 | 3.92 (1.30–12.00) | 0.017 | |
Values are presented as number (%). Bold values are statistically significant.
LoD = limit of detection, SCORAD = SCORing Atopic Dermatitis, VOC = volatile organic compound, aOR = adjusted odds ratio, CI = confidence interval.
aχ2; Children with “negative” SCORADs vs. “positive” SCORADs (children with SCORAD = 0 was considered as “negative,” and children with SCORAD score 1 or higher as “positive” for SCORADs). Adjusted for sex, BMI z-score, prematurity or/and low birth weight, allergen sensitization (no vs. yes), secondary smoking, current asthma, and current allergic rhinitis using a generalized linear regression with the logit function.
bLow level and high level were subdivided based on median values.
Contribution of each VOC to atopic dermatitis and SCORAD
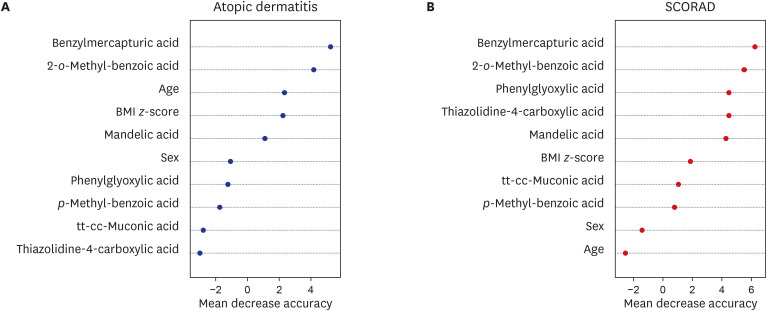
We used a random forest analysis to evaluate the strength of the contribution of each metabolite in predicting atopic dermatitis based on ISAAC and positive SCORAD results (Fig. 1). The results indicated that benzylmercapturic acid and o-methylbenzoic acid had the strongest effects in predicting the presence of atopic dermatitis, and positive SCORADs.
Fig. 1. Random forest analysis of the contribution of different urinary VOC metabolites to presence of atopic dermatitis (A) and SCORADs (B).
SCORAD = SCORing Atopic Dermatitis, VOC = volatile organic compound, BMI = body mass index.
DISCUSSION
The major finding of this study is that the urinary concentrations of benzylmercapturic acid (toluene metabolite), mandelic acid (benzene metabolite), and p-methylbenzoic acid (xylene metabolite) were significantly related with presence of atopic dermatitis diagnosed by ISAAC and atopic dermatitis symptoms represented as SCORAD results. To the best of our knowledge, this is the first study to identify positive associations of individual urinary levels of VOC metabolites with atopic dermatitis in children. Understanding the roles of indoor air pollutants in children is especially important because they spend most of their time indoors.17,18 Thus, controlling indoor air quality by reducing the usage of products that emit VOCs, such as toluene, benzene, and xylene, might decrease the risk of atopic dermatitis in children. Importantly, we directly measured urinary VOC metabolites, and these data provide a better indication of the personal burden of VOCs than air samples. Previous environmental and validation studies documented that concentrations of these VOC metabolites and ambient concentrations are highly correlated, and accuracy values generally meet the criteria for bioanalytical method validation.19,20
The burden of atopic dermatitis has increased in Korea3 and worldwide.21,22 during recent decades, and several previous studies investigated the associations of exposure to indoor air pollution with atopic dermatitis.5,6,10,18,23 However, fewer studies have investigated the association of indoor air pollution with SCORAD results.7,24 Furthermore, most of these previous studies estimated VOC exposures using ambient air samples.7,10 Some studies reported no direct links between ambient air levels of VOCs and atopic dermatitis.10,24 This may be because passive air monitoring does not consider personal variations, the chronic course of atopic dermatitis, and exposure to outdoor pollutants. An examination using individual monitoring overcomes these limitations.11 Thus, our study based on measurements of VOC metabolites provides strong support for the hypothesis that presence and symptoms of atopic dermatitis is associated with exposure to VOCs.25
Leipzig's Allergy Risk Study reported that excretions of toluene metabolites (based on urine measurements) were associated with atopic dermatitis.6 In agreement, we also found that benzylmethylmercapturic acid (a toluene metabolite) and ethyl-benzene (a mandelic acid metabolite) were significantly associated with the prevalence of atopic dermatitis and related symptoms. This is in concordance with earlier studies that measured VOCs using passive air sampling.24,26,27 However, we found that mandelic acid appeared to be less important than toluene based on ISAAC and SCORAD results. This could be because of the low concentrations of the VOCs detected in urine samples of our findings compared with previous studies,28,29 which intended to measure urinary VOC concentrations to estimate the extent of exposures, rather than a true non-association. Likewise, our random forest analysis indicated that the toluene metabolite had the strongest effect based on ISAAC and SCORAD results. Toluene is a well-known VOC, and previous studies reported its level correlated with atopic dermatitis and SCORADs.7,23 Previous molecular studies that investigated the association between atopic dermatitis and VOCs suggested that toluene exposure upregulated mRNA expression of thymic stromal lymphopoietin, a cytokine that plays a key role in the pathogenesis of atopic dermatitis in keratinocytes.30,31 This result implies a strong association between toluene exposure and symptoms occurred in atopic dermatitis.32
The main strength of our study is that we used measurements of urinary VOC metabolites to estimate exposure to VOCs. These measurements using biomonitoring methods provide more accurate access to individual exposures and biological burdens, because children may differ in routes of exposure, environments where they spend most of their time, and individual health conditions. Previous data, which relied on measurements of VOCs in ambient air focused on occupationally exposed individuals, but ambient concentrations of air contaminants should be considered as a surrogate of true exposures. We believe that our findings using biomonitoring of VOCs hereby offer meaningful information for association with presence of atopic dermatitis and symptoms represented as and SCORAD results. In addition, physicians performed the diagnosis of atopic dermatitis using the ISAAC and scaled the severity of disease using the SCORAD index. Children with positive SCORAD index values did not concur with those diagnosed as atopic dermatitis based on ISAAC questionnaires. This might possibly because the diagnosis depended on parents’ answers. For this reason, we considered that notification of clinical signs/symptoms of atopic dermatitis by separate analyses performed by physicians has its own significance.
However, our measurements of urinary metabolites of VOCs do not indicate actual exposures to these compounds. A further longitudinal study of this topic is thus necessary. In addition, our results should be interpreted with caution because the sample size was small and limited to children of a single city in Korea. Selection or participation bias may exist as subjects that agreed to participation and properly completed the questionnaires were enrolled, and parents with specific health concerns were more likely to participate. Our study does not include measurement of VOC exposure before and at different phases of atopic dermatitis’s occurrence due to the nature of the cross-sectional study design. Causal relationships might be established through further prospective and controlled studies. Furthermore, the small sample size and undetectable levels of urinary VOC metabolites imposes limitations to our study, and large-scale data sets or reference levels for VOC metabolites might be helpful in establishing validated safety levels in children.
In summary, we documented an association between exposure to VOCs and atopic dermatitis in children based on measurements of their urinary levels of VOC metabolites. Our results imply that the urinary levels of VOC metabolites in children reflect exposure to these compounds and the incidence of atopic dermatitis. Our findings have shown associations between increased urinary levels of the mandelic acid and benzylmercapturic acid (metabolites of ethylbenzene and toluene) with presence and symptoms of atopic dermatitis in children. These findings elucidate that specific VOCs might be attributable to atopic dermatitis.
Footnotes
Funding: This study was supported by grants from the Seongnam Atopy Project for Children Happiness (SAP2017) cohort by the Seongnam City Government, and the National Research Foundation of Korea (NRF) grant funded by the Korea government (NRF2020R1F1A1076452), Republic of Korea. The funders had no role in the data analysis or the decision to publish.
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Ha EK, Park D, Jee HM, Shin YH, Han MY.
- Data curation: Ha EK, Kim JH, Lee SW, Jee HM, Han MY.
- Formal analysis: Kim JH, Lee SW, Han MY.
- Funding acquisition: Lee SW, Jee HM, Han MY.
- Investigation: Ha EK, Lee E, Jee HM, Shin YH.
- Methodology: Kim JH, Park D, Lee E.
- Supervision: Lee E, Shin YH, Han MY.
- Validation: Lee E.
- Writing - original draft: Ha EK, Park D, Han MY.
- Writing - review & editing: Ha EK, Kim JH, Lee E, Jee HM, Shin YH, Han MY.
SUPPLEMENTARY MATERIALS
LoDs and levels of VOC metabolites used to separate the low VOC and high VOC groupsa
Analysis of the association tVOC metabolites with SCORAD
References
- 1.Ahn K. The role of air pollutants in atopic dermatitis. J Allergy Clin Immunol. 2014;134(5):993–999. doi: 10.1016/j.jaci.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 2.Yon DK, An J, Ha EK, Jee HM, Izuhara K, Ono J, et al. Serum periostin is negatively correlated with exposure to formaldehyde and volatile organic compounds in children. Allergy Asthma Immunol Res. 2018;10(6):716–721. doi: 10.4168/aair.2018.10.6.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee KS. Trends in prevalence of allergic diseases in Korean children: how and why? Clin Exp Pediatr. 2020;63(7):263–264. doi: 10.3345/cep.2020.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JT. Review of epidemiological studies on air pollution and health effects in children. Clin Exp Pediatr. 2021;64(1):3–11. doi: 10.3345/cep.2019.00843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herbarth O, Fritz GJ, Rehwagen M, Richter M, Röder S, Schlink U. Association between indoor renovation activities and eczema in early childhood. Int J Hyg Environ Health. 2006;209(3):241–247. doi: 10.1016/j.ijheh.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Rolle-Kampczyk UE, Rehwagen M, Diez U, Richter M, Herbarth O, Borte M. Passive smoking, excretion of metabolites, and health effects: results of the Leipzig’s Allergy Risk Study (LARS) Arch Environ Health. 2002;57(4):326–331. doi: 10.1080/00039890209601416. [DOI] [PubMed] [Google Scholar]
- 7.Kim J, Kim H, Lim D, Lee YK, Kim JH. Effects of indoor air pollutants on atopic dermatitis. Int J Environ Res Public Health. 2016;13(12):1220. doi: 10.3390/ijerph13121220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi H, Schmidbauer N, Sundell J, Hasselgren M, Spengler J, Bornehag CG. Common household chemicals and the allergy risks in pre-school age children. PLoS One. 2010;5(10):e13423. doi: 10.1371/journal.pone.0013423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Araki A, Kanazawa A, Kawai T, Eitaki Y, Morimoto K, Nakayama K, et al. The relationship between exposure to microbial volatile organic compound and allergy prevalence in single-family homes. Sci Total Environ. 2012;423:18–26. doi: 10.1016/j.scitotenv.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 10.Kwon JH, Kim E, Chang MH, Park EA, Hong YC, Ha M, et al. Indoor total volatile organic compounds exposure at 6 months followed by atopic dermatitis at 3 years in children. Pediatr Allergy Immunol. 2015;26(4):352–358. doi: 10.1111/pai.12393. [DOI] [PubMed] [Google Scholar]
- 11.Nurmatov UB, Tagiyeva N, Semple S, Devereux G, Sheikh A. Volatile organic compounds and risk of asthma and allergy: a systematic review. Eur Respir Rev. 2015;24(135):92–101. doi: 10.1183/09059180.00000714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ha EK, Kim JH, Lee SW, Jee HM, Shin YH, Baek HS, et al. Atopic dermatitis: correlation of severity with allergic sensitization and eosinophilia. Allergy Asthma Proc. 2020;41(6):428–435. doi: 10.2500/aap.2020.41.200067. [DOI] [PubMed] [Google Scholar]
- 13.Kunz B, Oranje AP, Labrèze L, Stalder JF, Ring J, Taïeb A. Clinical validation and guidelines for the SCORAD index: consensus report of the European Task Force on Atopic Dermatitis. Dermatology. 1997;195(1):10–19. doi: 10.1159/000245677. [DOI] [PubMed] [Google Scholar]
- 14.Ahn SH, Yoon W, Lee SY, Shin HS, Lim MY, Nam YD, et al. Effects of Lactobacillus pentosus in children with allergen-sensitized atopic dermatitis. J Korean Med Sci. 2020;35(18):e128. doi: 10.3346/jkms.2020.35.e128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wahn U, Warner J, Simons FE, de Benedictis FM, Diepgen TL, Naspitz CK, et al. IgE antibody responses in young children with atopic dermatitis. Pediatr Allergy Immunol. 2008;19(4):332–336. doi: 10.1111/j.1399-3038.2007.00643.x. [DOI] [PubMed] [Google Scholar]
- 16.Shin HS, Ahn HS, Lee BH. Determination of thiazolidine-4-carboxylates in urine by chloroformate derivatization and gas chromatography-electron impact mass spectrometry. J Mass Spectrom. 2007;42(9):1225–1232. doi: 10.1002/jms.1255. [DOI] [PubMed] [Google Scholar]
- 17.Franklin PJ. Indoor air quality and respiratory health of children. Paediatr Respir Rev. 2007;8(4):281–286. doi: 10.1016/j.prrv.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Youm S, Lee E, Lee J. Environmental and dietary factors to be checked for treatment of atopic dermatitis in rural children. Clin Exp Pediatr. 2021;64(12):661–663. doi: 10.3345/cep.2021.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sabatini L, Barbieri A, Indiveri P, Mattioli S, Violante FS. Validation of an HPLC-MS/MS method for the simultaneous determination of phenylmercapturic acid, benzylmercapturic acid and o-methylbenzyl mercapturic acid in urine as biomarkers of exposure to benzene, toluene and xylenes. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;863(1):115–122. doi: 10.1016/j.jchromb.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 20.Janasik B, Jakubowski M, Wesołowski W, Kucharska M. Unmetabolized VOCs in urine as biomarkers of low level occupational exposure. Int J Occup Med Environ Health. 2010;23(1):21–26. doi: 10.2478/v10001-010-0003-x. [DOI] [PubMed] [Google Scholar]
- 21.Mohn CH, Blix HS, Halvorsen JA, Nafstad P, Valberg M, Lagerløv P. Incidence trends of atopic dermatitis in infancy and early childhood in a nationwide prescription registry study in Norway. JAMA Netw Open. 2018;1(7):e184145. doi: 10.1001/jamanetworkopen.2018.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams H, Stewart A, von Mutius E, Cookson W, Anderson HR, International Study of Asthma and Allergies in Childhood (ISAAC) Phase One and Three Study Groups Is eczema really on the increase worldwide? J Allergy Clin Immunol. 2008;121(4):947–954.e15. doi: 10.1016/j.jaci.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Kim K. Influences of environmental chemicals on atopic dermatitis. Toxicol Res. 2015;31(2):89–96. doi: 10.5487/TR.2015.31.2.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim EH, Kim S, Lee JH, Kim J, Han Y, Kim YM, et al. Indoor air pollution aggravates symptoms of atopic dermatitis in children. PLoS One. 2015;10(3):e0119501. doi: 10.1371/journal.pone.0119501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pénard-Morand C, Raherison C, Charpin D, Kopferschmitt C, Lavaud F, Caillaud D, et al. Long-term exposure to close-proximity air pollution and asthma and allergies in urban children. Eur Respir J. 2010;36(1):33–40. doi: 10.1183/09031936.00116109. [DOI] [PubMed] [Google Scholar]
- 26.Kim J, Kim EH, Oh I, Jung K, Han Y, Cheong HK, et al. Symptoms of atopic dermatitis are influenced by outdoor air pollution. J Allergy Clin Immunol. 2013;132(2):495–498.e1. doi: 10.1016/j.jaci.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 27.Lee JH, Lee HS, Park MR, Lee SW, Kim EH, Cho JB, et al. Relationship between indoor air pollutant levels and residential environment in children with atopic dermatitis. Allergy Asthma Immunol Res. 2014;6(6):517–524. doi: 10.4168/aair.2014.6.6.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohashi Y, Mamiya T, Mitani K, Wang B, Takigawa T, Kira S, et al. Simultaneous determination of urinary hippuric acid, o-, m- and p-methylhippuric acids, mandelic acid and phenylglyoxylic acid for biomonitoring of volatile organic compounds by gas chromatography–mass spectrometry. Anal Chim Acta. 2006;566(2):167–171. [Google Scholar]
- 29.Choi W, Kim S, Baek YW, Choi K, Lee K, Kim S, et al. Exposure to environmental chemicals among Korean adults-updates from the second Korean National Environmental Health Survey (2012-2014) Int J Hyg Environ Health. 2017;220(2 Pt A):29–35. doi: 10.1016/j.ijheh.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Yu G, Zhang Y, Wang X, Sai L, Bo C, Yeo AJ, et al. Thymic stromal lymphopoietin (TSLP) and Toluene-diisocyanate-induced airway inflammation: alleviation by TSLP neutralizing antibody. Toxicol Lett. 2019;317:59–67. doi: 10.1016/j.toxlet.2019.09.021. [DOI] [PubMed] [Google Scholar]
- 31.Park A, Lee E, Park H, Park MN, Lee J, Song KB, et al. Innate type 2 response to Aspergillus fumigatus in a murine model of atopic dermatitis-like skin inflammation. J Korean Med Sci. 2021;36(40):e261. doi: 10.3346/jkms.2021.36.e261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee H, Park JB, Bae HC, Ryu WI, Shin JJ, Son SW. Toluene induces early growth response-1 dependent thymic stromal lymphopoietin expression in human keratinocytes. Mol Cell Toxicol. 2016;12(3):273–279. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
LoDs and levels of VOC metabolites used to separate the low VOC and high VOC groupsa
Analysis of the association tVOC metabolites with SCORAD