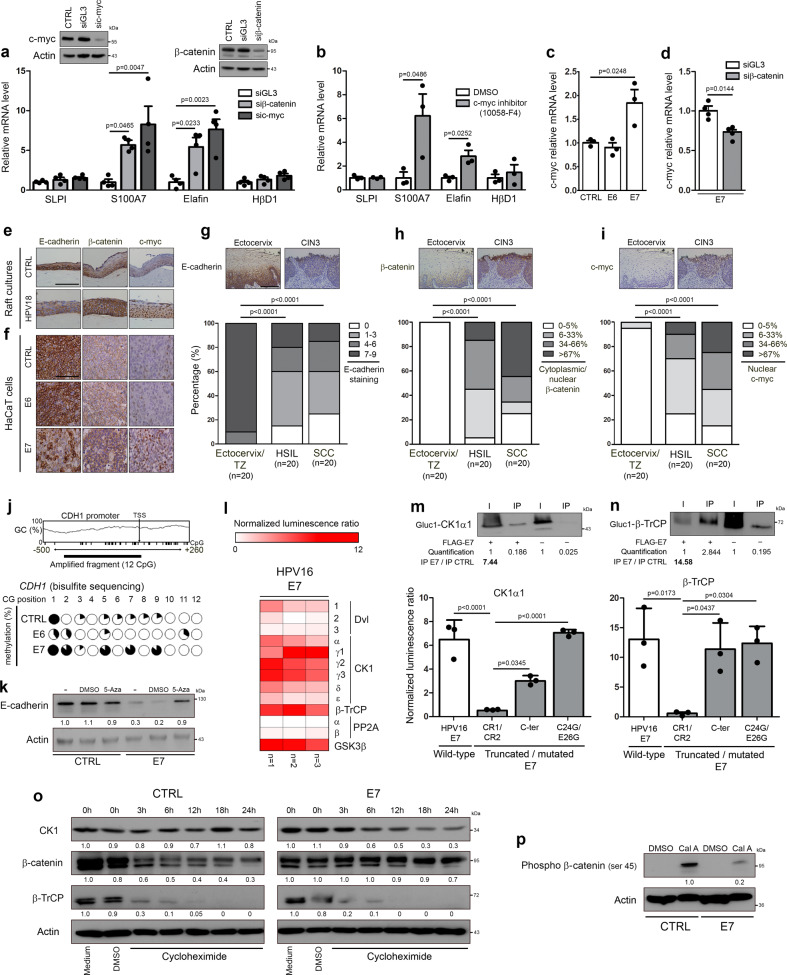
Fig. 4. E7 viral oncoprotein inhibits constitutive innate peptide expression (elafin and S100A7) through promoting β-catenin stabilization/signaling and subsequent c-myc expression.
The reactivation of so called “constitutive” peptide (SLPI, S100A7, elafin and HβD1) expression was analyzed by RT-qPCR in HPV16 E6E7-positive cells transfected with siRNA targeting c-myc or β-catenin a or treated with 40 µM 10058-F4 b. Cells transfected with control siRNA (siGL3) or treated with DMSO were used as negative controls. Each experiment was normalized to the amount of HPRT mRNA from the same sample. Results represent the means ± SEM of three b or four a independent experiments. The level of c-myc mRNA was determined by RT-qPCR in control, HPV16 E6 and E7-transduced cells c. Results represent the means ± SEM of three independent experiments. The effect of knockdown of β-catenin on c-myc expression in HPV16 E7-positive cells was assessed by RT-qPCR d. Results represent the means ± SEM of four independent experiments. e Representative pictures of control and HPV18-positive organotypic raft culture sections stained for E-cadherin, β-catenin and c-myc. f Representative examples of anti-E-cadherin, β-catenin and c-myc immunoreactivities displayed by control, E6-positive and E7-positive keratinocytes. Anti-E-cadherin g, anti-β-catenin h and anti-c-myc immunostainings i in both HPV-negative specimens [ectocervix/transformation zone (TZ), n = 20] and HPV-positive (pre)neoplastic lesions (HSIL, n = 20; SCC, n = 20). Note the reduced expression of E-cadherin associated with cytoplasmic/nuclear β-catenin immunoreactivity observed in HPV-positive tissues. Regarding c-myc, its expression was almost exclusively detected in HPV-positive (pre)neoplastic lesions. j The methylation status of E-cadherin gene (CDH1) promoter in control, HPV16 E6 and E7-transduced cells was assessed by bisulfite genomic sequencing. The variation in GC content within CDH1 promoter as well as the percentage of methylation of each individual CpG island contained in the PCR fragment analyzed by bisulfite sequencing are shown. k The reactivation of hypermethylated CDH1 gene was determined by Western blot following 5-aza-deoxycytidine (5-Aza, 5 µM) treatment. l Binding of protein members of the β-catenin degradation complex with HPV16 E7 oncoprotein was assessed by Gaussia princeps luciferase complementation assay (GPCA). Heatmap representing the score (normalized luminescence ratio) of each analyzed protein for potential interaction with HPV E7 is shown. Three independent experiments were performed. The interactions between HPV16 E7 oncoprotein and CK1α1 m or β-TrCP n were validated by both Co-IP and GPCA using the truncated/mutated forms of E7. Results represent the means ± SEM of three independent experiments. o CK1, β-catenin and β-TrCP stability in both control and HPV16 E7-transduced cells following treatment with cycloheximide (100 µM). p CK1-dependent phosphorylation of β-catenin (ser45) in both control and E7-positive cells following treatment with calyculin A (50 nM). The scale bar represents 100 μm. P values were determined using one-way ANOVA followed by Dunnett’s multiple comparison post-hoc test a, c, two-sided unpaired t-tests b, d, χ2 test g, h, i and one-way ANOVA followed by Bonferroni post-hoc test m, n. The Western blot and co-IP analyses were independently performed three times and twice, respectively. One representative experiment is shown. Source data are provided as a Source Data file.