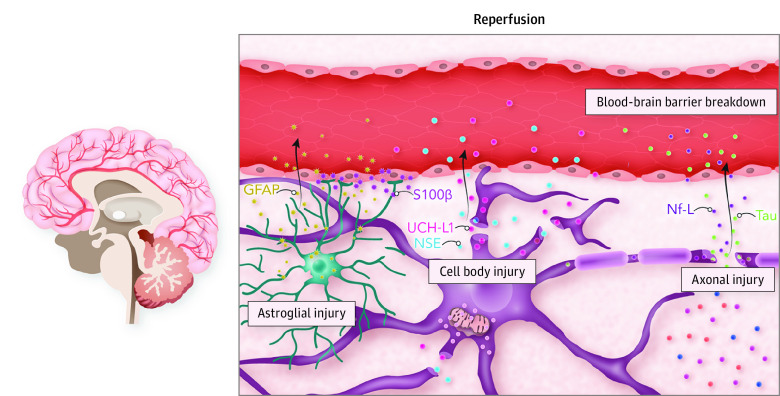
Figure 4. The Neurovascular Unit and Brain Injury Biomarker Release.
The neurovascular unit represents the principal anatomical and functional unit of the brain parenchyma where complex interplay occurs among neurons, the cerebral microvasculature, and surrounding glial cells to maintain homeostasis. The microvasculature is composed of the blood-brain barrier, which is partially formed by adjoining projections from astrocytes. Surrounding neuron cell bodies give rise to myelinated axons, which conduct signal transduction and facilitate communication with distinct anatomical locations in the brain. Following return of spontaneous circulation, ischemia-reperfusion injury pathophysiology occurs, and widespread injury across the neurovascular unit is reflected in the release of brain injury biomarkers into the bloodstream, which is facilitated by blood-brain barrier breakdown. Biomarkers reflecting astrocyte injury include glial fibrillary acidic protein (GFAP) and serum 100 calcium-binding protein β (S100β). Neuron cell body injury is reflected by release of neuron-specific enolase (NSE) and ubiquitin carboxyl hydrolase L1 (UCH-L1). In addition, axonal injury is reflected by release of neurofilament light (Nf-L) and tau. As such, the relative concentrations of the various biomarkers seen in the bloodstream can allude to signatures of damage to the neurovascular unit and its specific components.