Graphical abstract
Keywords: Therapeutic nucleic acids, Nucleic acid nanotechnology, Dna origami, Non-viral vector, Vaccine
Abstract
With numerous recent advances, the field of therapeutic nucleic acid nanotechnology is now poised for clinical translation supported by several examples of FDA-approved nucleic acid nanoformulations including two recent mRNA-based COVID-19 vaccines. Within this rapidly growing field, a new subclass of nucleic acid therapeutics called nucleic acid nanoparticles (NANPs) has emerged in recent years, which offers several unique properties distinguishing it from traditional therapeutic nucleic acids. Key unique aspects of NANPs include their well-defined 3D structure, their tunable multivalent architectures, and their ability to incorporate conditional activations of therapeutic targeting and release functions that enable diagnosis and therapy of cancer, regulation of blood coagulation disorders, as well as the development of novel vaccines, immunotherapies, and gene therapies. However, non-consolidated research developments of this highly interdisciplinary field create crucial barriers that must be overcome in order to impact a broader range of clinical indications. Forming a consortium framework for nucleic acid nanotechnology would prioritize and consolidate translational efforts, offer several unifying solutions to expedite their transition from bench-to-bedside, and potentially decrease the socio-economic burden on patients for a range of conditions. Herein, we review the unique properties of NANPs in the context of therapeutic applications and discuss their associated translational challenges.
1. Introduction
The pressing need for novel technologies that improve drug delivery and advance vaccines and immunotherapies is becoming increasingly apparent because (i) over 7000 genetic diseases have already been identified from genome-wide association studies in humans [1]; (ii) as revealed by the ongoing COVID-19 pandemic [2], there is a large number of latent viral and bacterial pathogens for which neither vaccines nor viable commercialization paths currently exist, due in part to a lack of incentive for the pharmaceutical industry; (iii) costs of cancer treatments remain inordinately high [3], even though a large number of cancers remain untreatable by current chemotherapeutics, antibodies, and cell-based therapeutics; and, perhaps most importantly, (iv) there is now a plethora of effective gene therapeutic modalities [4] that offers viable treatment pathways for each of the preceding classes of disease. Not surprisingly, therapeutic nucleic acids (TNAs), which are a relatively new class of drugs, face similar challenges of affordability to health care providers and patients and a lack of suitable technologies to facilitate in vivo, target-specific delivery of personalized formulations with controlled immunogenicity and minimal toxicity [5], [6], [7].
Specifically, it is now evident that TNAs offer an emerging category of therapies that are growing rapidly in their capabilities, and despite the fact that the initial conception of TNAs was to mimic components of biological systems, they have demonstrated several unique properties distinguishing them from small molecules and biologics. These unique properties have resulted in TNA separation into a discrete class of drug products for the purposes of regulatory evaluation and approval by the FDA [8], [9], [10]. As traditionally known to the scientific community, TNAs include antisense oligonucleotides (ASOs), RNAi inducers (siRNAs, miRNAs, shRNAs, etc.), aptamers, ribozymes, immunomodulatory oligonucleotides, messenger RNAs (mRNAs), and gene editing tools such as CRISPR/Cas9 [11]. The main hurdles with clinical use of these TNAs are their delivery to target cells and tissues, and instability in biological matrices. Several of these hurdles have been overcome for some indications using nanotechnology carriers such as lipid and polymeric nanoparticles, as well as specific chemical modifications of TNAs. Most traditional TNAs and TNAs formulated using nanotechnology carriers are currently in clinical trials, with several having reached the clinic by obtaining US Food and Drug Administration (FDA) approvals [4]. However, the focus of this manuscript is on the recently emerged subclass of TNAs that includes nanomaterials made nearly exclusively of nucleic acids (RNA, DNA and their chemical analogs), which are distinguished by (1) their programmable, precise 3D virus-like or other tertiary structure that is unlike conventional, disordered nucleic acid therapeutics; (2) their multivalency that can incorporate controlled, stoichiometric numbers of receptor targeting ligands such as aptamers or peptides or therapeutic cargo loading of siRNAs or ASOs; and (3) their conditional activation that can incorporate specific receptor/pathway targeting or therapeutic cargo release through, for example, Boolean logic gates, which, taken together, define this unique subclass of therapeutic nucleic acid nanotechnology as Nucleic Acid Nanoparticles, or NANPs.
2. Nucleic acid nanotechnology
Unlike traditional TNAs, when molecular engineering approaches are applied to the same starting nucleic acid materials (RNA, DNA, DNA-RNA hybrids, and chemical analogs), they can be programmed to self-assemble reproducibly into discrete, monodisperse NANPs that have well defined secondary and tertiary structure as well as a higher degree of chemical complexity, manifest in their size, aspect ratios, composition, dimensionality, etc. [12], [13]. For example, one-dimensional rodlike particles, two-dimensional planar sheets, or three-dimensional icosahedral virus-like particles of precise dimensions on the several to 100 nm scale can be fabricated [12], [14]. These novel nanostructures made of nucleic acids typically have higher molecular weight and anionic charge, greater number of distinct chemical components, and broader spectrum of unique physicochemical and biological attributes that otherwise cannot be achieved with traditional TNAs. Moreover, conditional activation can also be incorporated to, for example, interconvert an inactive tubular one-dimensional NANP to an active planar two-dimensional NANP based on intracellular cues [15]. As such, NANPs meet nanotechnology definitions of both the National Nanotechnology Initiative and the US FDA [16], [17]. Moreover, following the rationale of the Oligonucleotide Safety Working Group formed by the Drug Information Association for the separation of TNA from biologics and small molecules [9], [10], it was hypothesized that NANPs represent a category of therapeutic products that share some similarity to TNAs, yet they are clearly also distinct from TNAs [18]. Unique hallmarks of NANPs are their structural and functional programmability, biocompatibility, and precise control over their synthesis and functionalization, which make them a promising next-generation drug delivery platform for modulating biological responses through organization of traditional TNA pathways (RNAi, splicing, gene editing, etc). Indeed, encoded NANPs’ distinct therapeutic modalities offer control over their architectural parameters and valency and can reach clinical scale at reasonable cost compared to lipid nanoparticles (LNPs), yet at drastically reduced manufacturing cost and timeline compared to other gene therapeutics such as adeno-associated virus (AAV). At the same time, NANPs maintain diverse functions, amongst which is their ability to regulate immunological responses through cytosolic sensors of nucleic acids (e.g., STING, MDA5, RIG-I, Pol III) and endosomal TLR pathways [13], [19], [20]. The inflammatory response through these receptors that the cells mount in response to traditional TNAs, leads to acute toxicities such as cytokine storm that limits clinical use of such materials. Unlike traditional TNAs, the macromolecular nature of NANPs makes them invisible to immune cells in that large size and anionic charge interfere with their interaction with the anionic cellular membrane, thereby preventing their uptake; these rather unique immunological properties have led to the rapid adoption of these nanomaterials for diverse biomedical applications [11], [21]. Such adoption has additionally accelerated in recent years as facile design and fabrication procedures have emerged [14], [22], [23], [24], [25] to rationally design NANPs capable of controlling trafficking, targeting, and molecular behavior in various human systems—by directly interacting with and reporting factors of disease to shut them down at their source—with NANP-regulated immunomodulation that can be tuned for beneficial therapeutic outcomes [26], [27], [28], [29].
A variety of NANP platforms is currently available to applied and basic science researchers. The high programmability of RNA and DNA, along with the availability of diverse chemical modifications, allow for tunable chemical and thermodynamic stability of NANPs [20]. This in turn further enhances the modularity and adaptability of this technology to a broad range of biomedical applications. At the level of individual NANP design, the architectural parameters, type of delivery carrier, and chemical makeup of NANPs determine their biological activity, their interactions with particular biological pathways, and their immunostimulatory properties, as has been shown in numerous recent studies [11], [13], [19], [20], [26], [27], [28], [30], [31], [32], [33], [34], [35], [36], [37], [38]. For example, the chemical composition of NANPs and the type of delivery carrier both define the intracellular compartmentalization of NANPs and their interactions with pattern recognition receptors, with RNA NANPs generally more immunostimulatory than their DNA counterparts [13], [20], [28], [35], [38].
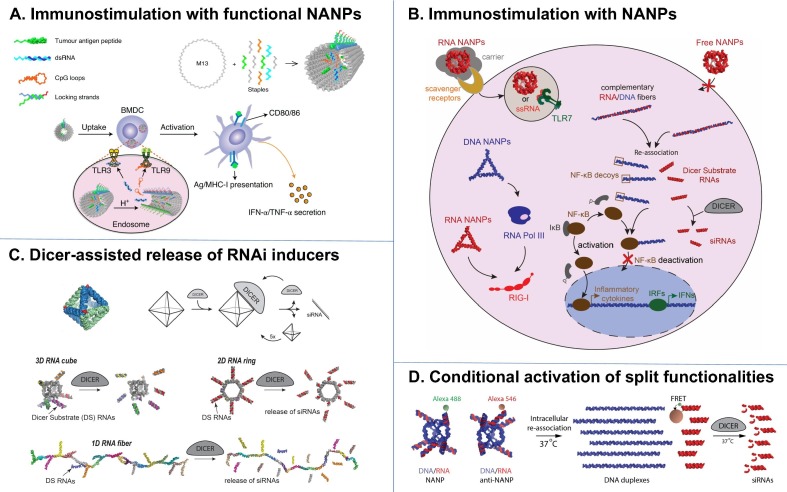
Current availability of diverse computational tools [14], [39], [40] allows for NANPs to be programmed to adopt nearly any geometry and size on the 10–100 nm scale [14], [24], and to be further functionalized chemically in a site-specific manner either using click chemistry or hybridization [41], [42]. Following this approach, a library of functional NANPs has been engineered for endowing cell targeting and intracellular trafficking of NANPs, as well as delivering gene therapeutic properties via the incorporation of peptides, aptamers, siRNAs, ASOs, and other chemical modalities including immunoprotective and serum stabilizing groups [12], [28], [29], [43], [44], [45], [46]. Two recent examples of promising applications of NANP technologies include their use in a cancer vaccines and immunotherapies [13], [15] and TNA delivery [27], [33], [45], [47], [48], [49], [50], [51], [52], respectively (Fig. 1 ). Efficient immunomodulation can be achieved by either using the NANPs with architectural parameters readily recognized by the PRRs upon NANPs’ intracellular delivery to the immune cells or by NANPs additionally functionalized with certain antigens or adjuvant molecules. For immunostimulation with functional NANPs, tumor antigen peptides were incorporated with adjuvant CpG and dsRNA motifs that were sequestered until site-specific, endosomal release within target dendritic cells (Fig. 1 A) [15]. In vitro and in vivo results demonstrated co-delivery of these payloads to elicit an antigen-specific CD8+ CTL response. On the other hand, by simply optimizing the size, shape and composition of NANPs delivered into the human immune cells with different delivery agents, one can regulate the extent of NANPs’ immunorecognition and tune the profile of induced cytokines [13], [19], [20], [35], [37], [38] (Fig. 1 B). For drug delivery, either scaffolding messenger RNA or programming short RNA oligos to assemble into the discrete nanostructures allows for production of NANPs that then can be co-formulated with Dicer Substrate RNAs to elicit efficient gene silencing through the Dicer-assisted recognition pathways both in vitro and in vivo (Fig. 1 C). Alternatively, interdependent, shape-switching NANPs can be engineered to conditionally activate embedded split functionalities only inside the diseased cells [31], [36], [53], [54], [55], [56] (Fig. 1 D). Taken together, these recent strategies demonstrate the ability to co-formulate multiple targeting and gene therapeutic modalities, as well as endow specific triggering and responsive properties in cellular pathways of interest. While significant fundamental work is still required to better characterize the efficacy of these formulations in vivo compared with standard, competing delivery technologies such as LNPs, together with a growing list of other therapeutic formulations and capabilities [29], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], NANPs offer promising avenues for further research into potentially clinically relevant modalities.
Fig. 1.
Overview of diverse NANP formulations and their biomedical applications. (A) DNA origami composed of a long single-stranded DNA folded with shorter, synthetic oligos can be used to formulate site-specific, responsive nanomaterials for cancer vaccines. Tumor antigens are co-formulated with RNA and DNA adjuvants, resulting in functional NANPs, which stimulate dendritic cells site-specifically for CTL activation. Figure reproduced from [15]. (B) NANPs architectural parameters and chemical compositions define specific immunorecognition upon their intracellular delivery [13], [35], [36]. (C) Messenger RNA scaffold or various NANPs, made of short oligos, can be co-formulated with therapeutic RNAi inducers to facilitate Dicer assisted release of siRNAs and consequent gene silencing [33], [45], [47]. Upper panel of figure reproduced from [47]. (D) Functionally interdependent NANPs can be designed for conditional activation of various split functionalities among which are RNAi, FRET, transcription, aptamers, etc [31].
Clinical indications for which NANPs are being developed include diagnosis and therapy of cancer, regulation of blood coagulation disorders, vaccines, immunotherapies, and gene therapies, to name a few [45], [69], [70]. Increased stability in biological matrix, polyvalency, multifunctionality and controlled properties, along with immunological quiescence – the properties distinguishing NANPs from traditional TNAs – make these biomedical applications possible and have moved NANPs from discovery towards clinical translation. The example of polyvalency includes the addition of diverse therapeutic oligonucleotides to the same backbone NANP such as the addition of an aptamer, an siRNA and an immunostimulatory CpG oligonucleotide to create a NANP capable of targeting a receptor of choice, downregulating gene expression and stimulating the immune system [21], [31], [32], [36], [60]. This multifunctionality is exemplified by NANPs that deliver aptamers performing an activating function and an “antidote” oligonucleotide that, when released, cancels the biological effect of the activating aptamer [57].
3. Funding therapeutic RNA and nanotechnology research
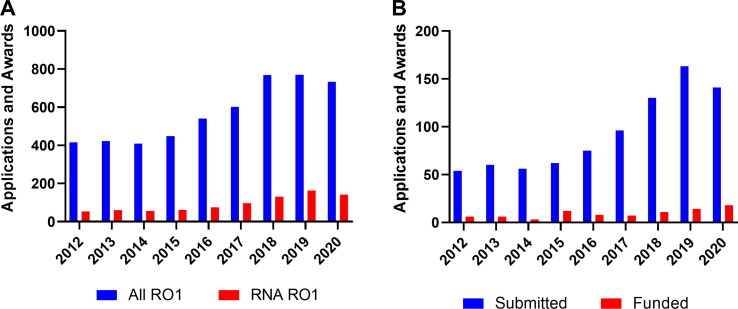
National Cancer Institute (NCI) has been funding nanotechnology for over 15 years. As part of this commitment, the institute established the NCI Alliance for Nanotechnology in Cancer program with multi-project Centers of Cancer Nanotechnology Excellence (CCNEs) at its core [71]. CCNEs operated successfully till 2020 [72] and produced a large body of scientific knowledge demonstrated through high impact publications. Equally important, they also produced applied technologies, which resulted in the formation of start-up companies that matured and commercialized these technologies. Current funding of nanotechnology at NCI occurs predominantly via the R01 mechanism—a ‘work horse’ of the NIH funding system. Overall, the number of incoming NCI nano R01 applications has been steadily increasing (415 new applications in 2012) and stabilized over 700 new applications per year in the last three years (Fig. 2 A). The majority of early cancer nanotechnology research was focused on the development of technology platforms, which, somewhat naively, were believed to be capable of supporting multiple therapeutic or diagnostic modalities. Over time, the functionality of designs and their correlation with target end-point applications increased. The delivery of TNAs in general and RNA in particular has been an important part of this research due to the critical need of preventing RNA deterioration in systemic delivery. The number of R01 applications associated with nanotechnology and RNA has grown significantly in the past decade (Fig. 2 A), reaching ∼150 submissions in 2020 alone. The portion of all NCI R01s related to RNA research has also increased over time: from 13% in 2012 to 19% in 2020, indicating the importance of nanotechnology approaches in RNA delivery.
Fig. 2.
NCI R01 nanotechnology-based applications submitted and awarded per fiscal year in the period of 2012–2020. (A) R01 applications associated with nanotechnology and RNA research (B) R01 applications associated with any nanotechnology and cancer research area. (B). The data were obtained using an internal NIH grant database and contains information on both new and resubmitted grants. Nanotechnology (A) submissions were identified using a search with the NIH RCDC (Research, Condition, and Disease Categorization) term of “nano AND RNA” “nano”, while nanotechnology submissions (B) used search term “nano”.
Several of these grants used nanoparticles (NPs) to deliver RNA-based cargo. For example, Bhujwalla et al. used biodegradable dextran based theranostic nanoparticles for delivery of siRNA for immunotherapy applications [73]. They were able to downregulate PD-L1 in tumors and demonstrated significant difference in the accumulation of these particles in tumors as compared to normal tissues. Lu, Camphausen et al. used a novel nanomaterial, ECO (1-aminoethylimino[bis(N-oleoylcysteinylaminoethyl) propionamide]), to deliver siRNA targeting DDR proteins to provide radiosensitization of GBM [74]. Treatment with ECO/siRNA NPs and radiation indicated DNA damage and, thus increased radiosensitivity in tumor cell lines. In vivo, intratumoral injection of these NPs with radiation resulted in a significant increase in survival compared with injection of NPs alone. Shi et al. developed a redox-responsive NP platform for effective delivery of p53-encoding synthetic messenger RNA (mRNA) [75]. The synthetic p53-mRNA NPs delayed the growth of p53-null hepatocellular carcinoma (HCC) and non-small cell lung cancer (NSCLC); it was also shown that p53 restoration improved the sensitivity of these tumor cells to mTOR inhibitors. RNA can be also a building block to develop NPs with therapeutic capabilities. Guo et al., has contributed to the field of therapeutic RNA nanotechnology by showing that RNA provides a high level of diversity in the design of NANPs and is also capable of tuning their properties in terms of PK/PD characteristics, biodistribution and immunogenicity [52], [76].
4. Translational hurdles
Despite numerous resource-intensive, rigorous investigations led by well-recognized, international research programs that have successfully demonstrated significant academic achievements in the design, formulation, synthesis, and proof-of-concept therapeutic applications of nucleic acid nanotechnology, the highly interdisciplinary landscape of clinical translation of NANPs and the limited funding and resources available to academic researchers for large-scale pre-clinical studies manifests in crucial gaps in both the breadth of scientific foundations and the existence of translational infrastructure. These issues, along with several engineering challenges, must still be overcome for NANPs to succeed in translational medicine. Future work will elucidate whether the added complexity of NANPs endows unique therapeutic efficacy characteristics beyond competing technologies, and how translational bottlenecks including cost of production scale-up and toxicity may be overcome.
4.1. Differences from traditional TNAs
Current knowledge about NANP properties suggests they are distinct from traditional TNAs, small molecules, and biologics, and that, therefore, these materials that stem from TNAs still form a separate, distinct category of drug product [18]. This notion highlights the urgent need for systematic evaluation of the current state of nucleic acid nanotechnology by academic, industrial, and regulatory scientists to identify and cover gaps using a harmonized, collaborative approach. Among the primary knowledge gaps impeding the field of therapeutic nucleic acid nanotechnology are, in our opinions, understanding the absorption, distribution, metabolism, excretion, toxicological properties, pharmacokinetics, and overall dependence of these parameters on individual NANP physicochemical properties (e.g., size, dimensionality, composition, chemical and thermodynamic stabilities), chosen therapeutic action (e.g., cell targeting, regulation of gene expression, immunostimulation), and complexation with other materials (e.g., lipids, polymers, inorganic materials) when delivery carriers are required. Extensive physicochemical characterization to identify attributes critical to both NANP safety and efficacy, along with the development of formulation, delivery, packaging, and bioanalytical methodologies, represent other areas of preclinical development with substantial gaps.
Moreover, the ability to chemically conjugate NANPs with peptides, nanobodies, antibodies, lipids, carbohydrates, and small molecules, leads to the creation of materials with a higher degree of organization and complexity, thus offering nearly limitless opportunities for their biomedical applications. A downside of this increased structural complexity, however, is a more tangled path to regulatory approval. The US FDA Office of Combination Products handles submissions of medical products containing more than one category of product (e.g., drugs and biologics) and coordinates the review between different Centers within the FDA based on both the product type and the mode of action (https://www.fda.gov/combination-products/guidance-regulatory-information). The safety assessment of combination products is currently performed according to the framework established for each individual product category present in the combination product [77]. This implies that a combination product containing, for example, antibody functionalized NANPs that are additionally decorated with siRNAs and small molecule drugs, would undergo the characterization and risk assessment typical of drugs, biologics, and nucleic acids together. Moreover, additional studies will likely be required to verify the adequacy of physicochemical characterization, bioanalytical methods, product performance models, and the effects of NANP size and charge on biodistribution and toxicity, which is commonly the case with all combination products containing nanotechnological components [77].
4.2. Interactions with the immune system
The diverse interactions of NANPs with the human immune system also remain obscure and currently represent one of the most significant barriers that must be overcome for successful translation of NANPs to the clinic as novel vaccines and therapeutics [78]. At the same time, these same uncharacterized and, therefore, unknown interactions may offer important clinical opportunities for these materials. While one might expect an immune response to NANPs that is similar to that of traditional TNAs, significant differences in immune interactions may result from NANPs’ unique structural and physicochemical features. Understanding how NANPs interact with the immune system will enable tailoring formulations for maximal therapeutic efficacy, while minimizing undesirable immunostimulation and adverse immune-mediated effects. Recent studies have already elucidated key factors about these interactions, including that type I and III interferons serve as reproducible biomarkers of NANP immunorecognition; the quality of the immune response to NANPs depends on their delivery into blood cells by a carrier via the endolysosomal pathway mediated by scavenger and Toll-like Receptors; plasmacytoid dendritic cells are the primary source of NANP-induced interferons in human PBMCs; and physicochemical characteristics appear to determine the magnitude of the immune response [13], [19], [28], [31], [33], [34], [35], [36], [53], [79]. These properties reveal the opportunity to explore nucleic acid nanotechnology for vaccines and immunotherapies, with NANPs to be designed explicitly as independent therapeutic entities, and highlight the potential safety concerns should such nanomaterials reach systemic circulation and activate blood leukocytes.
4.3. Technological hurdles
Among technological hurdles that can potentially impede NANP clinical translation is the lack of standardized methods for their physicochemical characterization with defined standards that can be used by individual researchers to compare against their custom-made NANP constructs. Another barrier is the absence of affordable techniques for industrial-scale manufacturing of pyrogen-free NANPs. While some progress has been made in scaling up the synthesis of DNA origami-based nanoassemblies [23], [24], their clinical-scale production in compliance with Good Manufacturing Practice (GMP) under established protocols that can easily be transferred from academia and biotechnology start-up companies to contract research and manufacturing organizations (CROs and CMOs) present an urgent and unmet need.
4.4. Logistical challenges: Workforce and costs
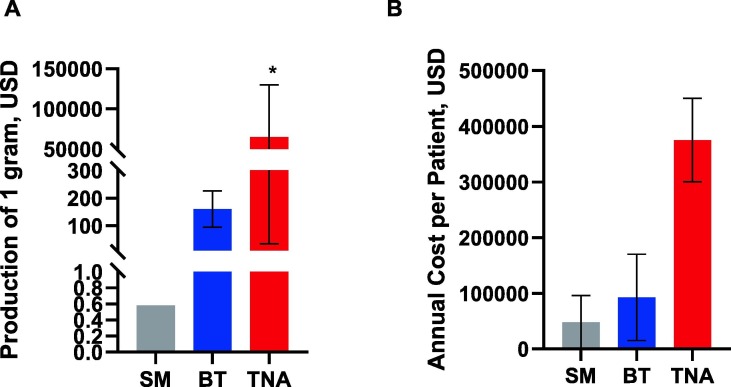
In addition, the translation of all novel and sophisticated nanomaterials such as NANPs, requires a suitably trained workforce. Therefore, educational plans and training programs aiming to prepare the next generation of scientists and industry workers trained for nucleic acid nanotechnology are needed. The average time and cost of developing any new drug entity and bringing it to market are estimated to be 10–15 years and $2.6 billion, respectively [80]. This estimate is in good agreement with actual resources invested by companies developing TNA therapeutics. For example, the development of the lipid-nanoparticle formulated siRNA (Onpattro® or Patisiran) started in 1998 and culminated with FDA approval in 2018; the overall cost of Patisiran’s 20-year journey from bench to the clinic cost its developer, Alnylam Pharmaceuticals, $2.5 billion [81]. Manufacturing costs and annual costs per patient of drug products may vary depending on the type of drug, and are influenced by other conditions (e.g., dose, treatment cycle, number of treatment cycles, availability of a generic version). TNAs stand out amongst other drug categories as being the costliest in terms of both manufacturing and financial burden for patients and insurance companies (Fig. 3 ).
Fig. 3.
Costs of drug products. Manufacturing costs (A) and the annual cost per patient (B) depend on the category of the drug product (small molecule (SM), biotechnology (BT) or therapeutic nucleic acid (TNA)). Annual costs also depend on dosing and treatment cycles. Each bar shows the mean cost at initial FDA approval; error bars show the range. Costs may vary significantly between individual products, and are influenced by the availability of a generic version of a drug. *Signifies non-GMP manufacturing; costs vary widely based on the type of TNA, length, chemical modifications, and purity. The data plotted are based on Refs. [81], [82], [83], [84], [85].
We speculate that translation of NANPs would require at a minimum the same amount of financial resources used to translate other TNAs, but we expect that, due to their higher complexity, NANP translational costs might be even higher. On the other hand, developmental costs have decreased due to advances in technological capabilities in characterization, synthesis, purification, and in vitro and in vivo cellular and animal models, amongst other areas, which might offset this increased cost.
It is also becoming apparent that even cumulative funding in all research areas supported by the NIH is substantially lower than that required to move a drug from discovery to approval. Thus, developers of NANPs face two global challenges, including formation of a rigorous and broad scientific foundation of the technology (e.g., carefully identified and executed research goals; sufficient funding; consolidated research developments), and establishment of the required translational infrastructure (e.g., a sufficiently skilled workforce; IP and protocol transfer; translational funding; sufficient clinical and economical value to pertinent stake-holders). Therefore, close coordination between academia, industry, CROs, CMOs, advisors from the FDA, and funding agencies is needed to identify all translational hurdles, and collectively prioritize efforts in order to overcome them.
5. Available and needed resources
Among the existing, federally funded resources that can be leveraged by the nucleic acid nanotechnology field is the US National Cancer Institute-funded Nanotechnology Characterization Laboratory (http://ncl.cancer.gov) that offers comprehensive preclinical characterization with a suite of assays to support the development of cancer nanomedicines. Moreover, a database sponsored by the NCI, The Cancer Nanotechnology Laboratory or caNanoLab (https://cananolab.nci.nih.gov/) is being expanded and maintained by the Frederick National Laboratory for Cancer Research to improve public access to data. The caNanoLab is designed to facilitate information sharing across the biomedical nanotechnology research community. It was originally developed as a collaboration between the NCI Center for Strategic Scientific Initiatives (CSSI) Office of Cancer Nanotechnology Research (OCNR) and the NCI Center for Biomedical Informatics and Information Technology (CBIIT). Attributing to users’ submission, CaNanoLab provides research information on biomedical nanomaterials and has quickly become a valuable tool for searching nanomaterials’ compositional information, physiochemical, in vitro, and in vivo characterizations, as well as access to protocols and publications relevant to cancer nanotechnology from the NCL and NCI Alliance for Nanotechnology in Cancer, respectively. Since its birth in 2006, more than 1700 sample data associated with the most well-studied nanomaterials in cancer nanotechnology have been submitted to caNanoLab, which are accessible to the public to review, leverage, and validate nanomaterials’ use in biomedicine.
The International Society of RNA Nanotechnology and Nanomedicine (https://www.isrnn.org/) could also play an important role in advancing the basic science of nucleic acid nanotechnology and supporting the educational goals of the consortium. Consolidating efforts by forming partnerships between researchers from other stakeholders such as the Institute for Clinical and Economic Review (https://icer-review.org/), the FDA Nanotechnology Task Force (https://www.fda.gov/science-research/nanotechnology-programs-fda/nanotechnology-task-force), the American Association of Pharmaceutical Scientists Advocacy (https://www.aaps.org/education-and-research/advocacy), and the Physician Consortium for Performance Improvement (https://www.thepcpi.org/) would further support translational efforts. Additional resources with more focus on nucleic acid nanotechnology are needed and require a contribution from both government and industry.
6. Conclusion and outlook into the future
In summary, the nucleic acid nanotechnology field has advanced tremendously in the design and synthesis of novel RNA- and DNA-based nanomaterials and NANPs. Properties unique to NANPs and not present in traditional TNAs such as improved stability in biological matrices, immunological quiescence, multifunctionality, and controlled biological activity [18], [86] clearly distinguish NANPs as a separate category of nucleic acid based therapeutics. Exciting proof of concept studies have already been published, ushering in the subsequent goal of advancing the clinical translation of this modality through optimization of NANP synthetic procedures and standardization of NANP characterization. Several initial steps have already been made in this direction. For example, ASTM International is working on two standard practices and one standard guide intended for the production and characterization of NANPs (https://www.astm.org/). In the long term, establishing comprehensive PK, PD, immunogenicity, and toxicology profiles of NANPs along with their large-scale production would bring these materials to clinical trials. Given constraints imposed on academics by limited financial resources, technical expertise,and academic focus on fundamental scientific questions, external partnerships with agencies such as the NIH and NCL will be essential to realize this aim.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
This study was supported in part by federal funds from the National Cancer Institute, the National Institutes of Health, under contracts HHSN261200800001E and 75N91019D00024 to M.A.D. and W.K. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. Research reported in this publication was also supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Numbers R01GM120487 and R35GM139587 (to K.A.A.). M.B. is grateful for funding to the National Institutes of Health R21-EB026008, R21-EB026008-S1, and R01-MH112694, the Office of Naval Research N00014-20-1-2084 and N00014-21-1-4013, the Army Research Office W911NF-13-D-0001, and the National Science Foundation CCF-1564025 and CBET-1729397.
Disclosure
The authors declare no financial conflict of interest regarding the content of this manuscript.
References
- 1.Mukherjee K. Care for Rare: Spotlight on Rare Diseases. Trends Pharmacol. Sci. 2019;40:227–228. doi: 10.1016/j.tips.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Panigaj M., Dobrovolskaia M.A., Afonin K.A. an immunotherapy odyssey and the rise of nucleic acid nanotechnology. Nanomedicine (Lond.) 2021;16(2021):1635–1640. doi: 10.2217/nnm-2021-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dieguez G., Ferro C., Pyenson B.S. A multi-year look at the cost burden of cancer care. Milliman Res. Rep. 2017;4:1–19. [Google Scholar]
- 4.Weng Y., Huang Q., Li C., Yang Y., Wang X., Yu J., Huang Y., Liang X.J. Improved Nucleic Acid Therapy with Advanced Nanoscale Biotechnology. Mol. Ther. Nucleic Acids. 2020;19:581–601. doi: 10.1016/j.omtn.2019.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Durymanov M., Reineke J. Non-viral Delivery of Nucleic Acids: Insight Into Mechanisms of Overcoming Intracellular Barriers. Front. Pharmacol. 2018;9:971. doi: 10.3389/fphar.2018.00971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kowalski P.S., Rudra A., Miao L., Anderson D.G. Delivering the Messenger: Advances in Technologies for Therapeutic mRNA Delivery. Mol. Ther. 2019;27:710–728. doi: 10.1016/j.ymthe.2019.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yin H., Kauffman K.J., Anderson D.G. Delivery technologies for genome editing. Nat. Rev. Drug Discov. 2017;16:387–399. doi: 10.1038/nrd.2016.280. [DOI] [PubMed] [Google Scholar]
- 8.Kornbrust D., Cavagnaro J., Levin A., Foy J., Pavco P., Gamba-Vitalo C., Guimond A. Oligo safety working group exaggerated pharmacology subcommittee consensus document. Nucleic Acid Ther. 2013;23:21–28. doi: 10.1089/nat.2012.0399. [DOI] [PubMed] [Google Scholar]
- 9.Cavagnaro J., Berman C., Kornbrust D., White T., Campion S., Henry S. Considerations for assessment of reproductive and developmental toxicity of oligonucleotide-based therapeutics. Nucleic Acid Ther. 2014;24:313–325. doi: 10.1089/nat.2014.0490. [DOI] [PubMed] [Google Scholar]
- 10.Schubert D., Levin A.A., Kornbrust D., Berman C.L., Cavagnaro J., Henry S., Seguin R., Ferrari N., Shrewsbury S.B. The Oligonucleotide Safety Working Group (OSWG) Nucleic Acid Ther. 2012;22:211–212. doi: 10.1089/nat.2012.0383. [DOI] [PubMed] [Google Scholar]
- 11.Johnson M.B., Chandler M., Afonin K.A. Nucleic acid nanoparticles (NANPs) as molecular tools to direct desirable and avoid undesirable immunological effects. Adv. Drug Deliv. Rev. 2021;173:427–438. doi: 10.1016/j.addr.2021.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Afonin K.A., Dobrovolskaia M.A., Church G., Bathe M. Opportunities, Barriers, and a Strategy for Overcoming Translational Challenges to Therapeutic Nucleic Acid Nanotechnology. ACS Nano. 2020;14:9221–9227. doi: 10.1021/acsnano.0c04753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong E., Halman J.R., Shah A.B., Khisamutdinov E.F., Dobrovolskaia M.A., Afonin K.A. Structure and Composition Define Immunorecognition of Nucleic Acid Nanoparticles. Nano Lett. 2018;18:4309–4321. doi: 10.1021/acs.nanolett.8b01283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veneziano R., Ratanalert S., Zhang K., Zhang F., Yan H., Chiu W., Bathe M. Designer nanoscale DNA assemblies programmed from the top down. Science. 2016;352:1534. doi: 10.1126/science.aaf4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu S., Jiang Q., Zhao X., Zhao R., Wang Y., Wang Y., Liu J., Shang Y., Zhao S., Wu T. A DNA nanodevice-based vaccine for cancer immunotherapy. Nat. Mater. 2021;20:421–430. doi: 10.1038/s41563-020-0793-6. [DOI] [PubMed] [Google Scholar]
- 16.National Nanotechnology Initiative, What is Nanotechnology?
- 17.FDA, Guidance for Indusrty: Considering Whether an FDA-Regulated Product Involves the Application of Nanotechnology, 2014.
- 18.Dobrovolskaia M.A. Self-assembled DNA/RNA nanoparticles as a new generation of therapeutic nucleic acids: immunological compatibility and other translational considerations, DNA and RNA. Nanotechnology. 2016;3:1–10. [Google Scholar]
- 19.Dobrovolskaia M.A., Afonin K.A. Use of human peripheral blood mononuclear cells to define immunological properties of nucleic acid nanoparticles. Nat. Protoc. 2020;15:3678–3698. doi: 10.1038/s41596-020-0393-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson M.B., Halman J.R., Miller D.K., Cooper J.S., Khisamutdinov E.F., Marriott I., Afonin K.A. The immunorecognition, subcellular compartmentalization, and physicochemical properties of nucleic acid nanoparticles can be controlled by composition modification. Nucleic Acids Res. 2020;48:11785–11798. doi: 10.1093/nar/gkaa908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo P. The emerging field of RNA nanotechnology. Nat. Nanotechnol. 2010;5:833–842. doi: 10.1038/nnano.2010.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Afonin K.A., Grabow W.W., Walker F.M., Bindewald E., Dobrovolskaia M.A., Shapiro B.A., Jaeger L. Design and self-assembly of siRNA-functionalized RNA nanoparticles for use in automated nanomedicine. Nat. Protoc. 2011;6:2022–2034. doi: 10.1038/nprot.2011.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Praetorius F., Kick B., Behler K.L., Honemann M.N., Weuster-Botz D., Dietz H. Biotechnological mass production of DNA origami. Nature. 2017;552:84–87. doi: 10.1038/nature24650. [DOI] [PubMed] [Google Scholar]
- 24.Shepherd T.R., Du R.R., Huang H., Wamhoff E.C., Bathe M. Bioproduction of pure, kilobase-scale single-stranded DNA. Sci. Rep. 2019;9:6121. doi: 10.1038/s41598-019-42665-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chandler M., Panigaj M., Rolband L.A., Afonin K.A. Challenges to optimizing RNA nanostructures for large scale production and controlled therapeutic properties. Nanomedicine (Lond.) 2020 doi: 10.2217/nnm-2020-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halman J.R., Kim K.T., Gwak S.J., Pace R., Johnson M.B., Chandler M.R., Rackley L., Viard M., Marriott I., Lee J.S., Afonin K.A. A cationic amphiphilic co-polymer as a carrier of nucleic acid nanoparticles (Nanps) for controlled gene silencing, immunostimulation, and biodistribution. Nanomedicine. 2020;23 doi: 10.1016/j.nano.2019.102094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nordmeier S., Ke W., Afonin K.A., Portnoy V. Exosome mediated delivery of functional nucleic acid nanoparticles (NANPs) Nanomedicine. 2020;30 doi: 10.1016/j.nano.2020.102285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Avila Y.I., Chandler M., Cedrone E., Newton H.S., Richardson M., Xu J., Clogston J.D., Liptrott N.J., Afonin K.A., Dobrovolskaia M.A. Induction of Cytokines by Nucleic Acid Nanoparticles (NANPs) Depends on the Type of Delivery Carrier. Molecules. 2021;26 doi: 10.3390/molecules26030652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Veneziano R., Moyer T.J., Stone M.B., Wamhoff E.C., Read B.J., Mukherjee S., Shepherd T.R., Das J., Schief W.R., Irvine D.J., Bathe M. Role of nanoscale antigen organization on B-cell activation probed using DNA origami. Nat. Nanotechnol. 2020;15:716–723. doi: 10.1038/s41565-020-0719-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bui M.N., Brittany Johnson M., Viard M., Satterwhite E., Martins A.N., Li Z., Marriott I., Afonin K.A., Khisamutdinov E.F. Versatile RNA tetra-U helix linking motif as a toolkit for nucleic acid nanotechnology. Nanomedicine. 2017;13:1137–1146. doi: 10.1016/j.nano.2016.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Halman J.R., Satterwhite E., Roark B., Chandler M., Viard M., Ivanina A., Bindewald E., Kasprzak W.K., Panigaj M., Bui M.N., Lu J.S., Miller J., Khisamutdinov E.F., Shapiro B.A., Dobrovolskaia M.A., Afonin K.A. Functionally-interdependent shape-switching nanoparticles with controllable properties. Nucleic Acids Res. 2017;45:2210–2220. doi: 10.1093/nar/gkx008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson M.B., Halman J.R., Satterwhite E., Zakharov A.V., Bui M.N., Benkato K., Goldsworthy V., Kim T., Hong E., Dobrovolskaia M.A., Khisamutdinov E.F., Marriott I., Afonin K.A. Programmable Nucleic Acid Based Polygons with Controlled Neuroimmunomodulatory Properties for Predictive QSAR Modeling. Small. 2017;13 doi: 10.1002/smll.201701255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rackley L., Stewart J.M., Salotti J., Krokhotin A., Shah A., Halman J.R., Juneja R., Smollett J., Lee L., Roark K., Viard M., Tarannum M., Vivero-Escoto J., Johnson P.F., Dobrovolskaia M.A., Dokholyan N.V., Franco E., Afonin K.A. RNA Fibers as Optimized Nanoscaffolds for siRNA Coordination and Reduced Immunological Recognition. Adv. Funct. Mater. 2018;28 doi: 10.1002/adfm.201805959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sajja S., Chandler M., Fedorov D., Kasprzak W.K., Lushnikov A., Viard M., Shah A., Dang D., Dahl J., Worku B., Dobrovolskaia M.A., Krasnoslobodtsev A., Shapiro B.A., Afonin K.A. Dynamic Behavior of RNA Nanoparticles Analyzed by AFM on a Mica/Air Interface. Langmuir. 2018;34:15099–15108. doi: 10.1021/acs.langmuir.8b00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hong E., Halman J.R., Shah A., Cedrone E., Truong N., Afonin K.A., Dobrovolskaia M.A. Toll-Like Receptor-Mediated Recognition of Nucleic Acid Nanoparticles (NANPs) in Human Primary Blood Cells. Molecules. 2019;24 doi: 10.3390/molecules24061094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ke W., Hong E., Saito R.F., Rangel M.C., Wang J., Viard M., Richardson M., Khisamutdinov E.F., Panigaj M., Dokholyan N.V., Chammas R., Dobrovolskaia M.A., Afonin K.A. RNA-DNA fibers and polygons with controlled immunorecognition activate RNAi, FRET and transcriptional regulation of NF-kappaB in human cells. Nucleic Acids Res. 2019;47:1350–1361. doi: 10.1093/nar/gky1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chandler M., Johnson M.B., Panigaj M., Afonin K.A. Innate immune responses triggered by nucleic acids inspire the design of immunomodulatory nucleic acid nanoparticles. NANPsCurr. Opin. Biotechnol. 2020;63:8–15. doi: 10.1016/j.copbio.2019.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson M.B., Halman J.R., Burmeister A.R., Currin S., Khisamutdinov E.F., Afonin K.A., Marriott I. Retinoic acid inducible gene-I mediated detection of bacterial nucleic acids in human microglial cells. J. Neuroinflamm. 2020;17:139. doi: 10.1186/s12974-020-01817-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Afonin K.A., Kasprzak W.K., Bindewald E., Kireeva M., Viard M., Kashlev M., Shapiro B.A. In silico design and enzymatic synthesis of functional RNA nanoparticles. Acc. Chem. Res. 2014;47:1731–1741. doi: 10.1021/ar400329z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jun H., Wang X., Parsons M.F., Bricker W.P., John T., Li S., Jackson S., Chiu W., Bathe M. Rapid prototyping of arbitrary 2D and 3D wireframe DNA origami. Nucleic Acids Res. 2021;49:10265–10274. doi: 10.1093/nar/gkab762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knappe G.A., Wamhoff E.-C., Read B.J., Irvine D.J., Bathe M. In Situ Covalent Functionalization of DNA Origami Virus-like Particles. ACS Nano. 2021;15:14316–14322. doi: 10.1021/acsnano.1c03158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wamhoff E.-C., Banal J.L., Bricker W.P., Shepherd T.R., Parsons M.F., Veneziano R., Stone M.B., Jun H., Wang X., Bathe M. Programming structured DNA assemblies to probe biophysical processes. Annu. Rev. Biophys. 2019;48:395–419. doi: 10.1146/annurev-biophys-052118-115259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Auvinen H., Zhang H., Nonappa A., Kopilow E.H., Niemelä S., Nummelin A., Correia H.A., Santos V., Linko M.A.K. Protein Coating of DNA Nanostructures for Enhanced Stability and Immunocompatibility. Adv. Healthc. Mater. 2017;6 doi: 10.1002/adhm.201700692. [DOI] [PubMed] [Google Scholar]
- 44.Ponnuswamy N., Bastings M.M.C., Nathwani B., Ryu J.H., Chou L.Y.T., Vinther M., Li W.A., Anastassacos F.M., Mooney D.J., Shih W.M. Oligolysine-based coating protects DNA nanostructures from low-salt denaturation and nuclease degradation. Nat. Commun. 2017;8:15654. doi: 10.1038/ncomms15654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saito R.F., Rangel M.C., Halman J.R., Chandler M., de Sousa Andrade L.N., Odete-Bustos S., Furuya T.K., Carrasco A.G.M., Chaves-Filho A.B., Yoshinaga M.Y., Miyamoto S., Afonin K.A., Chammas R. Simultaneous silencing of lysophosphatidylcholine acyltransferases 1–4 by nucleic acid nanoparticles (NANPs) improves radiation response of melanoma cells. Nanomedicine. 2021;36 doi: 10.1016/j.nano.2021.102418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ke W., Afonin K.A. Exosomes as natural delivery carriers for programmable therapeutic nucleic acid nanoparticles (NANPs) Adv. Drug Deliv. Rev. 2021:113835. doi: 10.1016/j.addr.2021.113835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Høiberg H.C., Sparvath S.M., Andersen V.L., Kjems J., Andersen E.S. An RNA Origami Octahedron with Intrinsic siRNAs for Potent Gene Knockdown. Biotechnol. J. 2019;14 doi: 10.1002/biot.201700634. [DOI] [PubMed] [Google Scholar]
- 48.Afonin K.A., Viard M., Koyfman A.Y., Martins A.N., Kasprzak W.K., Panigaj M., Desai R., Santhanam A., Grabow W.W., Jaeger L., Heldman E., Reiser J., Chiu W., Freed E.O., Shapiro B.A. Multifunctional RNA nanoparticles. Nano Lett. 2014;14:5662–5671. doi: 10.1021/nl502385k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shu Y., Pi F., Sharma A., Rajabi M., Haque F., Shu D., Leggas M., Evers B.M., Guo P. Stable RNA nanoparticles as potential new generation drugs for cancer therapy. Adv. Drug Deliv. Rev. 2014;66:74–89. doi: 10.1016/j.addr.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rychahou P., Haque F., Shu Y., Zaytseva Y., Weiss H.L., Lee E.Y., Mustain W., Valentino J., Guo P., Evers B.M. Delivery of RNA nanoparticles into colorectal cancer metastases following systemic administration. ACS Nano. 2015;9:1108–1116. doi: 10.1021/acsnano.5b00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shu D., Li H., Shu Y., Xiong G., Carson W.E., Haque F., Xu R., Guo P. Systemic Delivery of Anti-miRNA for Suppression of Triple Negative Breast Cancer Utilizing RNA Nanotechnology. ACS Nano. 2015 doi: 10.1021/acsnano.5b02471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jasinski D., Haque F., Binzel D.W., Guo P. Advancement of the Emerging Field of RNA Nanotechnology. ACS Nano. 2017;11:1142–1164. doi: 10.1021/acsnano.6b05737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Afonin K.A., Viard M., Kagiampakis I., Case C.L., Dobrovolskaia M.A., Hofmann J., Vrzak A., Kireeva M., Kasprzak W.K., KewalRamani V.N., Shapiro B.A. Triggering of RNA interference with RNA-RNA, RNA-DNA, and DNA-RNA nanoparticles. ACS Nano. 2015;9:251–259. doi: 10.1021/nn504508s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Afonin K.A., Desai R., Viard M., Kireeva M.L., Bindewald E., Case C.L., Maciag A.E., Kasprzak W.K., Kim T., Sappe A., Stepler M., Kewalramani V.N., Kashlev M., Blumenthal R., Shapiro B.A. Co-transcriptional production of RNA-DNA hybrids for simultaneous release of multiple split functionalities. Nucleic Acids Res. 2014;42:2085–2097. doi: 10.1093/nar/gkt1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Afonin K.A., Viard M., Martins A.N., Lockett S.J., Maciag A.E., Freed E.O., Heldman E., Jaeger L., Blumenthal R., Shapiro B.A. Activation of different split functionalities on re-association of RNA-DNA hybrids. Nat. Nanotechnol. 2013;8:296–304. doi: 10.1038/nnano.2013.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Panigaj M., Johnson M.B., Ke W., McMillan J., Goncharova E.A., Chandler M., Afonin K.A. Aptamers as Modular Components of Therapeutic Nucleic Acid Nanotechnology. ACS Nano. 2019;13:12301–12321. doi: 10.1021/acsnano.9b06522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Krissanaprasit A., Key C., Fergione M., Froehlich K., Pontula S., Hart M., Carriel P., Kjems J., Andersen E.S., LaBean T.H. Genetically Encoded, Functional Single-Strand RNA Origami: Anticoagulant. Adv. Mater. 2019;31 doi: 10.1002/adma.201808262. [DOI] [PubMed] [Google Scholar]
- 58.Krissanaprasit A., Key C., Fergione M., Froehlich K., Pontula S., Hart M., Carriel P., Kjems J., Andersen E.S., LaBean T.H. Genetically encoded, functional single-strand RNA origami: anticoagulant. Adv. Mater. 2019;31:1808262. doi: 10.1002/adma.201808262. [DOI] [PubMed] [Google Scholar]
- 59.Jiang Q., Liu S., Liu J., Wang Z.G., Ding B. Rationally designed DNA-origami nanomaterials for drug delivery in vivo. Adv. Mater. 2019;31:1804785. doi: 10.1002/adma.201804785. [DOI] [PubMed] [Google Scholar]
- 60.Jiang Q., Song C., Nangreave J., Liu X., Lin L., Qiu D., Wang Z.-G., Zou G., Liang X., Yan H. DNA origami as a carrier for circumvention of drug resistance. J. Am. Chem. Soc. 2012;134:13396–13403. doi: 10.1021/ja304263n. [DOI] [PubMed] [Google Scholar]
- 61.Zhang Q., Jiang Q., Li N., Dai L., Liu Q., Song L., Wang J., Li Y., Tian J., Ding B. DNA origami as an in vivo drug delivery vehicle for cancer therapy. ACS Nano. 2014;8:6633–6643. doi: 10.1021/nn502058j. [DOI] [PubMed] [Google Scholar]
- 62.Zhao S., Tian R., Wu J., Liu S., Wang Y., Wen M., Shang Y., Liu Q., Li Y., Guo Y. A DNA origami-based aptamer nanoarray for potent and reversible anticoagulation in hemodialysis. Nat. Commun. 2021;12:1–10. doi: 10.1038/s41467-020-20638-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu J., Song L., Liu S., Jiang Q., Liu Q., Li N., Wang Z.-G., Ding B. A DNA-based nanocarrier for efficient gene delivery and combined cancer therapy. Nano Lett. 2018;18:3328–3334. doi: 10.1021/acs.nanolett.7b04812. [DOI] [PubMed] [Google Scholar]
- 64.Douglas S.M., Bachelet I., Church G.M. A logic-gated nanorobot for targeted transport of molecular payloads. Science. 2012;335:831–834. doi: 10.1126/science.1214081. [DOI] [PubMed] [Google Scholar]
- 65.Li S., Jiang Q., Liu S., Zhang Y., Tian Y., Song C., Wang J., Zou Y., Anderson G.J., Han J.-Y. A DNA nanorobot functions as a cancer therapeutic in response to a molecular trigger in vivo. Nat. Biotechnol. 2018;36:258–264. doi: 10.1038/nbt.4071. [DOI] [PubMed] [Google Scholar]
- 66.Halley P.D., Lucas C.R., McWilliams E.M., Webber M.J., Patton R.A., Kural C., Lucas D.M., Byrd J.C., Castro C.E. Daunorubicin-loaded DNA origami nanostructures circumvent drug-resistance mechanisms in a leukemia model. Small. 2016;12:308–320. doi: 10.1002/smll.201502118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Halley P.D., Lucas C.R., McWilliams E.M., Webber M.J., Patton R.A., Kural C., Lucas D.M., Byrd J.C., Castro C.E., Origami D.N.A. Daunorubicin-Loaded DNA Origami Nanostructures Circumvent Drug-Resistance Mechanisms in a Leukemia Model (Small 3/2016) Small. 2016;12 doi: 10.1002/smll.201502118. 307–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kearney C.J., Lucas C.R., O'Brien F.J., Castro C.E., Origami D.N.A. Folded DNA-Nanodevices that can direct and interpret cell behavior. Adv. Mater. 2016;28:5509–5524. doi: 10.1002/adma.201504733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Binzel D.W., Li X., Burns N., Khan E., Lee W.J., Chen L.C., Ellipilli S., Miles W., Ho Y.S., Guo P. Thermostability, Tunability, and Tenacity of RNA as Rubbery Anionic Polymeric Materials in Nanotechnology and Nanomedicine-Specific Cancer Targeting with Undetectable Toxicity. Chem. Rev. 2021;121:7398–7467. doi: 10.1021/acs.chemrev.1c00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ke W., Afonin K.A. Exosomes as natural delivery carriers for programmable therapeutic nucleic acid nanoparticles (NANPs) Adv. Drug Deliv. Rev. 2021;176 doi: 10.1016/j.addr.2021.113835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hartshorn C.M., Russell L.M., Grodzinski P. National Cancer Institute Alliance for nanotechnology in cancer-Catalyzing research and translation toward novel cancer diagnostics and therapeutics. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019;11 doi: 10.1002/wnan.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grodzinski P. NCI Centers of Cancer Nanotechnology Excellence (CCNEs) - A full story to set the record straight. J. Control. Release. 2019;309:341–342. doi: 10.1016/j.jconrel.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 73.Pacheco-Torres J., Penet M.F., Krishnamachary B., Mironchik Y., Chen Z., Bhujwalla Z.M. PD-L1 siRNA Theranostics With a Dextran Nanoparticle Highlights the Importance of Nanoparticle Delivery for Effective Tumor PD-L1 Downregulation. Front. Oncol. 2020;10 doi: 10.3389/fonc.2020.614365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee J.A., Ayat N., Sun Z., Tofilon P.J., Lu Z.R., Camphausen K. Improving Radiation Response in Glioblastoma Using ECO/siRNA Nanoparticles Targeting DNA Damage Repair. Cancers (Basel) 2020;12 doi: 10.3390/cancers12113260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kong N., Tao W., Ling X., Wang J., Xiao Y., Shi S., Ji X., Shajii A., Gan S.T., Kim N.Y., Duda D.G., Xie T., Farokhzad O.C., Shi J. Synthetic mRNA nanoparticle-mediated restoration of p53 tumor suppressor sensitizes p53-deficient cancers to mTOR inhibition. Sci. Transl. Med. 2019;11 doi: 10.1126/scitranslmed.aaw1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guo S., Xu C., Yin H., Hill J., Pi F., Guo P. Tuning the size, shape and structure of RNA nanoparticles for favorable cancer targeting and immunostimulation. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020;12 doi: 10.1002/wnan.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tyner K.M., Zheng N., Choi S., Xu X., Zou P., Jiang W., Guo C., Cruz C.N. How Has CDER Prepared for the Nano Revolution? A Review of Risk Assessment, Regulatory Research, and Guidance Activities. AAPS J. 2017;19:1071–1083. doi: 10.1208/s12248-017-0084-6. [DOI] [PubMed] [Google Scholar]
- 78.Descotes J. Immunotoxicology: role in the safety assessment of drugs. Drug Saf. 2005;28:127–136. doi: 10.2165/00002018-200528020-00004. [DOI] [PubMed] [Google Scholar]
- 79.Bila D., Radwan Y., Dobrovolskaia M.A., Panigaj M., Afonin K.A. The Recognition of and Reactions to Nucleic Acid Nanoparticles by Human Immune Cells. Molecules. 2021;26 doi: 10.3390/molecules26144231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.DiMasi J.A., Grabowski H.G., Hansen R.W. Innovation in the pharmaceutical industry: New estimates of R&D costs. J. Health Econ. 2016;47:20–33. doi: 10.1016/j.jhealeco.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 81.Herper M. Alnylam Prices First Gene Silencing Drug At $450,000 Per Patient, But Offers Money-Back Guarantee. Forbes. 2018;10 [Google Scholar]
- 82.Edelman B. Explaining the cost of biotech therapies. Biotechnol. Healthc. 2004;1:37–41. [PMC free article] [PubMed] [Google Scholar]
- 83.Roy A. Biologic medicines: the biggest drive of rising drug prices. Forbes. 2019;3 [Google Scholar]
- 84.Stein C.A., Castanotto D. FDA-Approved Oligonucleotide Therapies in 2017. Mol. Ther. 2017;25:1069–1075. doi: 10.1016/j.ymthe.2017.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sun G., Riggs A.D. A Simple and Cost-Effective Approach for In Vitro Production of Sliced siRNAs as Potent Triggers for RNAi. Mol. Ther. Nucleic Acids. 2017;8:345–355. doi: 10.1016/j.omtn.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dobrovolskaia M.A., Bathe M. Opportunities and challenges for the clinical translation of structured DNA assemblies as gene therapeutic delivery and vaccine vectors. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021;13 doi: 10.1002/wnan.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]