Figure 3.
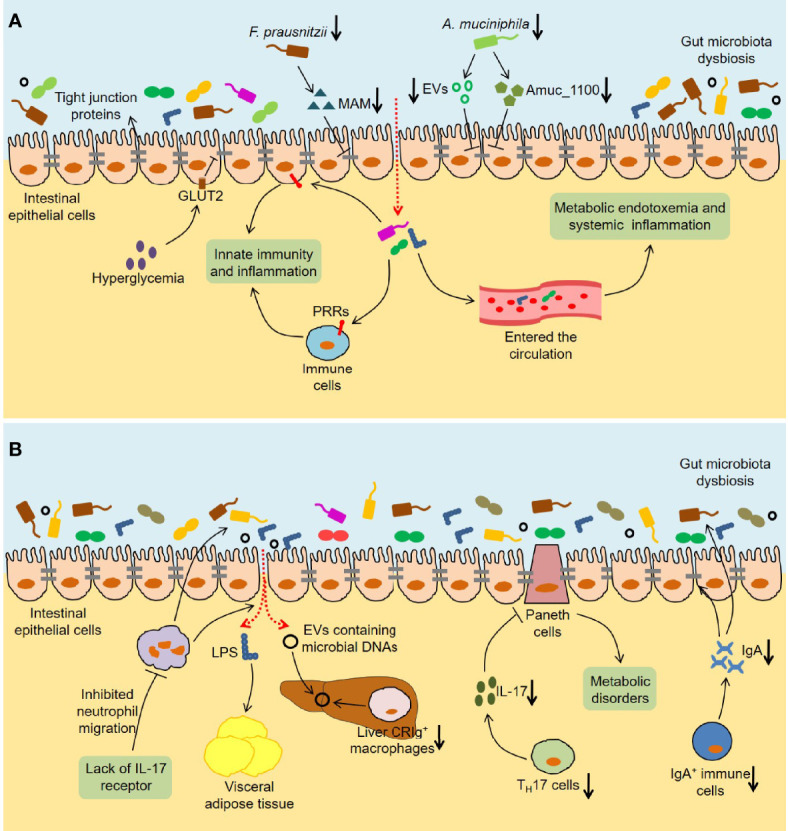
Immune and inflammatory responses induced by the gut microbiota in T2DM. (A) The intestinal barrier is disturbed in T2DM. Hyperglycemia disrupts the integrity of tight and adherent junctions by GLUT2. F. prausnitzii abundance and its metabolite MAM levels are downregulated in db/db mice, which results in damaged intestinal barrier by reducing ZO-1 expression. Analogously, A. muciniphila abundance and the levels of A. muciniphila-derived EVs and Amuc_1100 are reduced in diabetic mice, which also disrupts intestinal barrier integrity. The increased intestinal permeability leads to the translocation of bacteria and bacterial products, which not only activates the innate immunity and inflammation in the gut, but also causes metabolic endotoxemia and systemic inflammation. (B) Multiple immune cells are involved in the influence of the gut microbiota on host metabolism. Lack of IL-17 receptor promotes intestinal dysbiosis and LPS translocation into visceral adipose tissue by inhibiting the migration of neutrophils in the intestinal mucosa. Reduced liver CRIg+ macrophages leads to an accumulation of EVs containing gut microbial DNAs in obesity, thereby promoting tissue inflammation and insulin resistance. The number of TH17 cells is reduced in the intestinal tissues of obese mice, which leads to metabolic disorders by regulating the functions of Paneth cells. Furthermore, HFD-fed mice show a decrease in IgA+ immune cell percentage and secretory IgA concentrations in colon tissues, which impairs glucose metabolism by increasing intestinal permeability and changing microbial composition. GLUT2, Glucose transporter 2; MAM, Microbial anti-inflammatory molecule; ZO-1, Zonula occludens-1; EVs, Extracellular vesicles; PRRs, Pattern recognition receptors; IL-17, Interleukin 17; LPS, Lipopolysaccharide; CRIg, Complement receptor of the immunoglobulin superfamily.
