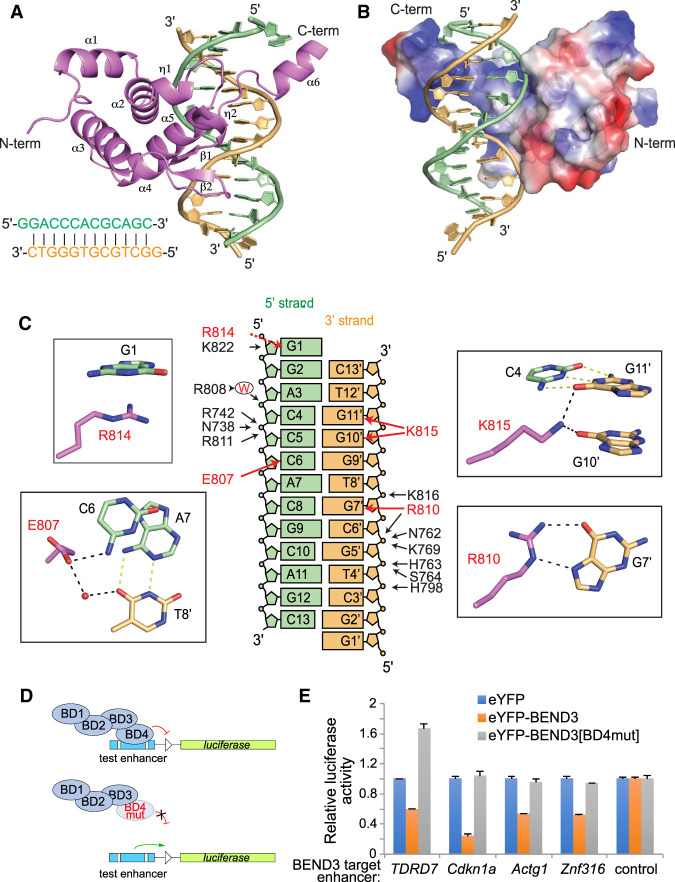
Figure 5.
Tertiary structure of the BEND3-BD4/DNA complex. (A) Tertiary structure of the BEND3-BD4 domain in complex with the DNA duplex shown in cartoon representation, with the BD4 domain in violet and the two strands of DNA complex in orange and pale green, respectively. The sequence of the 13-mer DNA duplex is shown at the bottom left. (B) Electrostatic surface representation of the BD4 domain in complex with DNA. (C) Summary of base contacts (red arrows) and sugar–phosphate interactions (black arrows) in the complex. The red capital W represents water. The details of the base interactions are shown in the black rectangle. G1 is stacking on R814. The side chain of E807 is hydrogen-bonded to the 4-NH2 of C6 in the 5′ strand and forms water-mediated interaction with T8′ in the 3′ strand. Both O6 of G10′ and G11′ form interactions with the side chain of K815. The Hoogsteen edge of G7′ forms hydrogen bonds with the side chain of R810. (D) Schematic for testing transcriptional regulation by wild-type and mutant BEND3 proteins. As guided by the structure, we mutated three BD4 residues involved in sequence-specific base contacts (E807V, R810L, and R814L). (E) Assays of transcriptional reporters of four BEND3 targets show that they are repressed by eYFP-BEND3, but not by eYFP-BEND3[BD4mut].