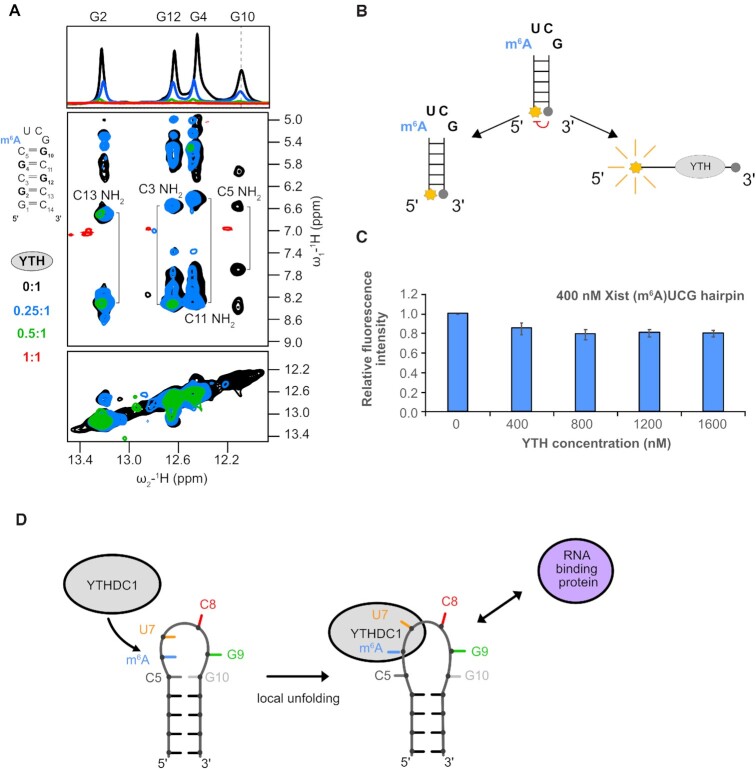
Figure 4.
YTH domain binding requires opening of the closing base pair of the (m6A)UCG tetraloop stem. (A) 1H 1D and 1H–1H NOESY spectra showing broadening imino proton resonances upon addition of YTH domain protein (black: apo RNA, blue: 0.25:1, green: 0.5:1 and red: 1:1). The G10 imino proton resonance (as indicated by the dashed line) disappears even in the presence of 0.25 molar ratio of YTH protein. (B) Schematic representation of the (m6A)UCG tetraloop hairpin with a fluorophore conjugated to the 5′ end and a quencher to the 3′ end. When the two probes are separated, there is emission of fluorescence intensity. (C) Bar plot showing the effect the YTH domain has on unwinding of the lower stem of the (m6A)UCG tetraloop hairpin at increasing concentration of protein. The RNA concentration was held constant at 400 nM. (D) Model illustrating the effects of m6A modification and YTHDC1 binding on the upper region of the Xist (m6A)UCG stem–loop. The conformational changes induced in the RNA upon YTH binding might modulate interactions with RNA-binding proteins or tertiary contacts involving the hairpin RNA.