Figure 3.
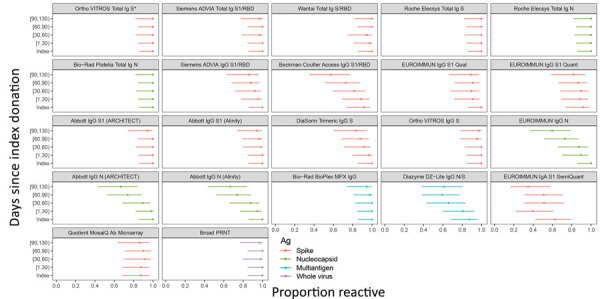
Proportion of donors with detectable severe acute respiratory syndrome coronavirus 2 antibodies in study of commercially available high-throughput assays for serosurveillance. In the longitudinal coronavirus disease convalescent plasma donor cohort, donations were sorted into time bins relative to index donation. Time bin labels on x-axis are denoted with brackets to indicate inclusive boundaries and parentheses to indicate exclusive boundaries. Donors who contributed >1 donation in a time bin contributed the fractional proportion reactive to the numerator and 1 to the denominator for estimation of proportion reactive in the time bin. Symbols indicate point estimates of proportion reactive, and bars indicate 95% CIs (Wilson score). Assays are described in Table 1. Each of the 24 donors had an index sample available. For time bins 1–29 days post index, n = 22 donors, n = 56 specimens; day 30–59, n = 19 donors, n = 45 specimens; day 60–89, n = 22 donors, n = 54 specimens; day 90+, n = 18 donors, n = 30 specimens. Ortho VITROS Total Ig anti-S reactivity was required for qualification of continued CCP donation and therefore shows 100% detection in all time bins by definition. Ab, antibody; Ag, antigen; N, nucleocapsid; PRNT, plaque reduction neutralization test; RBD, receptor binding domain; S, spike protein.
