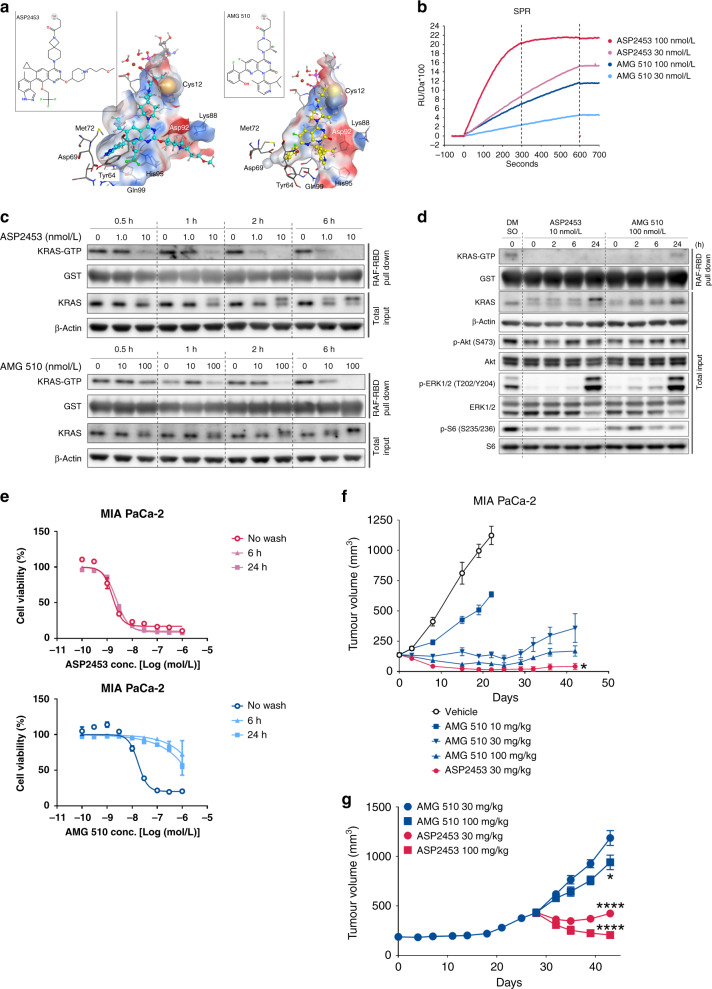
Fig. 6. Comparison of ASP2453 and AMG 510 in vitro and in vivo.
a Chemical structures and binding modes of ASP2453 (left) and AMG 510 (right). Chemical structures are shown in the insets. ASP2453, AMG 510, GDP, Mg2+ ion, and water molecules bound to the Mg2+ ion are shown as ball-and-stick figures and other atoms are shown as sticks. Each element is represented by a different colour (white: hydrogen, cyan or grey: carbon, blue: nitrogen, red: oxygen, yellow: sulfur). The colours of the protein surfaces are based on electrostatic potential (blue: positive, red: negative, white: neutral). For clarity, non-polar hydrogen atoms have been omitted and the protein surfaces at the front of ASP2453 and AMG 510 are hidden. b SPR sensorgram results for the association of ASP2453 and AMG 510 to KRAS G12C-GDP protein. c Time course of KRAS activation and KRAS mobility shift in MIA PaCa-2 cells. MIA PaCa-2 cells were treated with ASP2453 or AMG 510 for the indicated period of time. KRAS activation and KRAS mobility shift were detected using a RAF-RBD pulldown assay and immunoblotting. d Time course of KRAS activation, KRAS mobility shift and downstream signals in MIA PaCa-2 cells after removal of ASP2453 and AMG 510. MIA PaCa-2 cells were treated with ASP2453 or AMG 510 for 2 h. After treatment, each well was washed three times with medium and incubated for 0, 2, 6 or 24 h. KRAS activation, KRAS mobility shift and downstream signals were detected using a RAF-RBD pulldown assay and immunoblotting. e Inhibitory activity of ASP2453 and AMG 510 on the proliferation of MIA PaCa-2 cells after washout (mean ± SD). f Anti-tumour activity of ASP2453 and AMG 510 in a subcutaneous KRAS G12C-mutated MIA PaCa-2 xenograft model (n = 5 mice per group, mean ± SEM). *P < 0.05, Dunnett’s multiple comparison test compared with AMG 510 (30 mg/kg)-treated group. g Anti-tumour activity of ASP2453 and AMG 510 in an AMG 510-resistant MIA PaCa-2 xenograft model (mean ± SEM). AMG 510 (30 mg/kg) was orally administered once a day for 28 days. Mice were randomised into four groups (n = 5) based on tumour volume and ASP2453 or AMG 510 was administered orally once a day. *P < 0.05, ****P < 0.0001, Dunnett’s multiple comparison test compared with AMG 510 (30 mg/kg)-treated group.