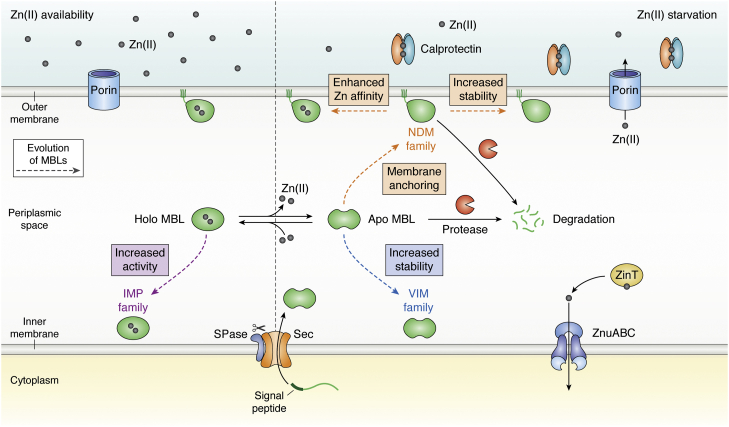
Figure 2.
Mechanisms of MBL evolution. Periplasmic Zn(II) levels depend on the passive diffusion through porins in Escherichia coli. In the presence of Zn(II), represented by gray spheres (left panel), the external concentration is in equilibrium with periplasmic Zn(II) levels (62, 63), and MBLs bind the cofactor and hydrolyze β-lactams. The dashed arrows highlight the different mechanism of MBL evolution (79, 130, 139). IMP families have evolved increasing their activity in response of antibiotic evolutionary pressure. When the innate immune response is active (right panel), neutrophils secrete calprotectin (CP) that chelates Zn(II) ions (43), reducing the periplasmic Zn(II) levels and activating expression of the Zn(II) uptake mechanisms to the E. coli cytosol (ZnuABC system and ZinT chaperone). MBLs lose the Zn(II) cofactor leading to formation of apo-MBLs. The nonmetallated form is susceptible to degradation in the periplasm by proteases (shown as Pac-Man-like orange objects) (44). Different mechanisms of evolution are indicated with dashed arrows of different colors, such as outer membrane anchoring (44), stabilization toward degradation, or enhancement of the Zn(II) affinity (15, 73). IMP, imipenemase; MBL, metallo-β-lactamase; SPase, signal peptidase.