Figure 1.
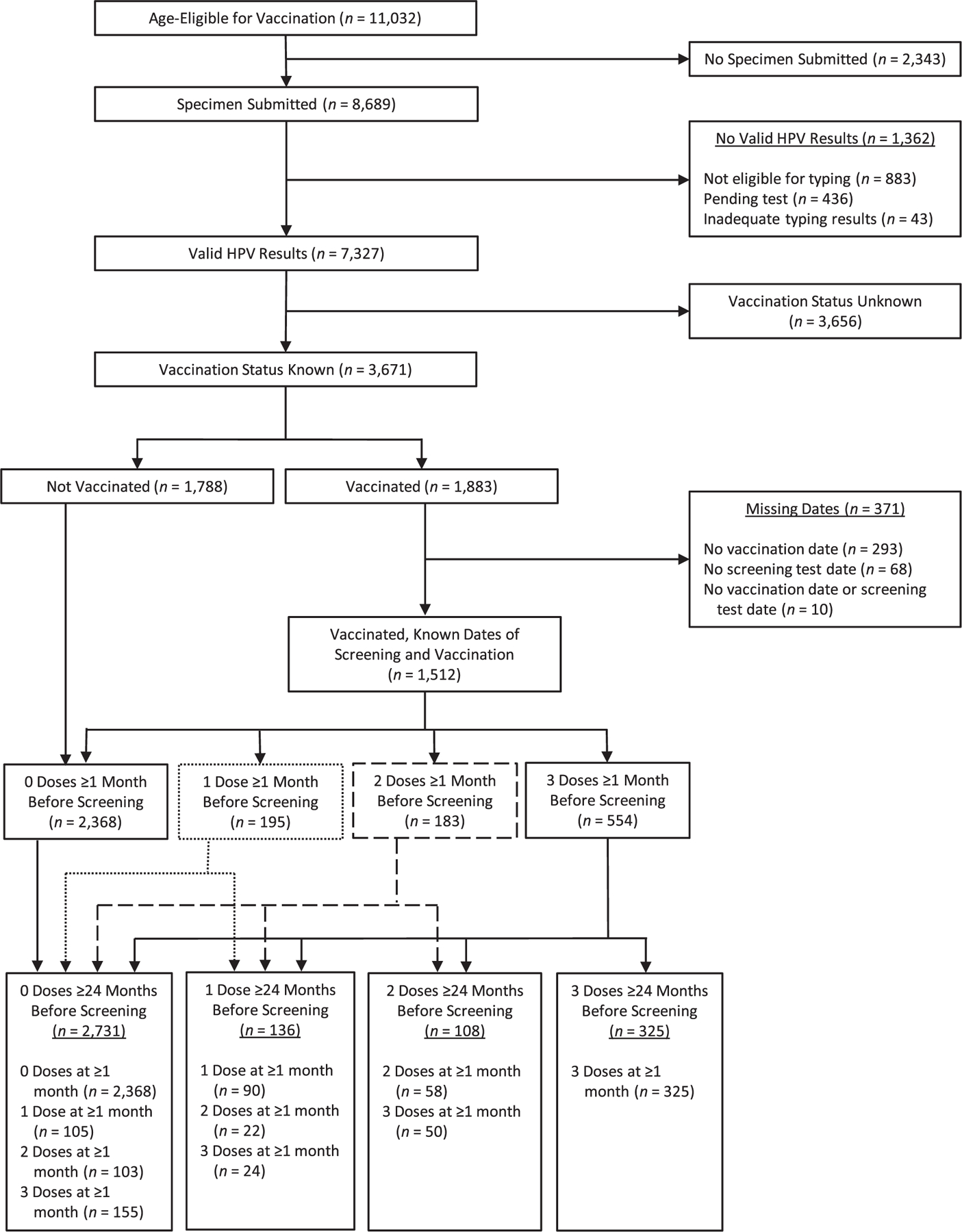
Inclusion of women with cervical intraepithelial neoplasia grades 2–3 and adenocarcinoma in situ in an analysis of human papillomavirus (HPV) vaccine effectiveness, HPV-IMPACT, United States, 2008–2014. The numbers adjacent to solid or broken lines show the number of women reassigned from dose groups ≥1 month before screening. For example, of the 183 women who received 2 doses of HPV vaccine ≥1 month before screening, 58 received 2 doses ≥24 months before screening and were assigned to the 2-dose group, 22 received 1 dose ≥24 months before screening and were reassigned to the 1-dose group, and 103 received both doses less than 24 months before screening and were reassigned to the 0-dose group. HPV-IMPACT, Human Papillomavirus Vaccine Impact Monitoring Project.
