Figure 3.
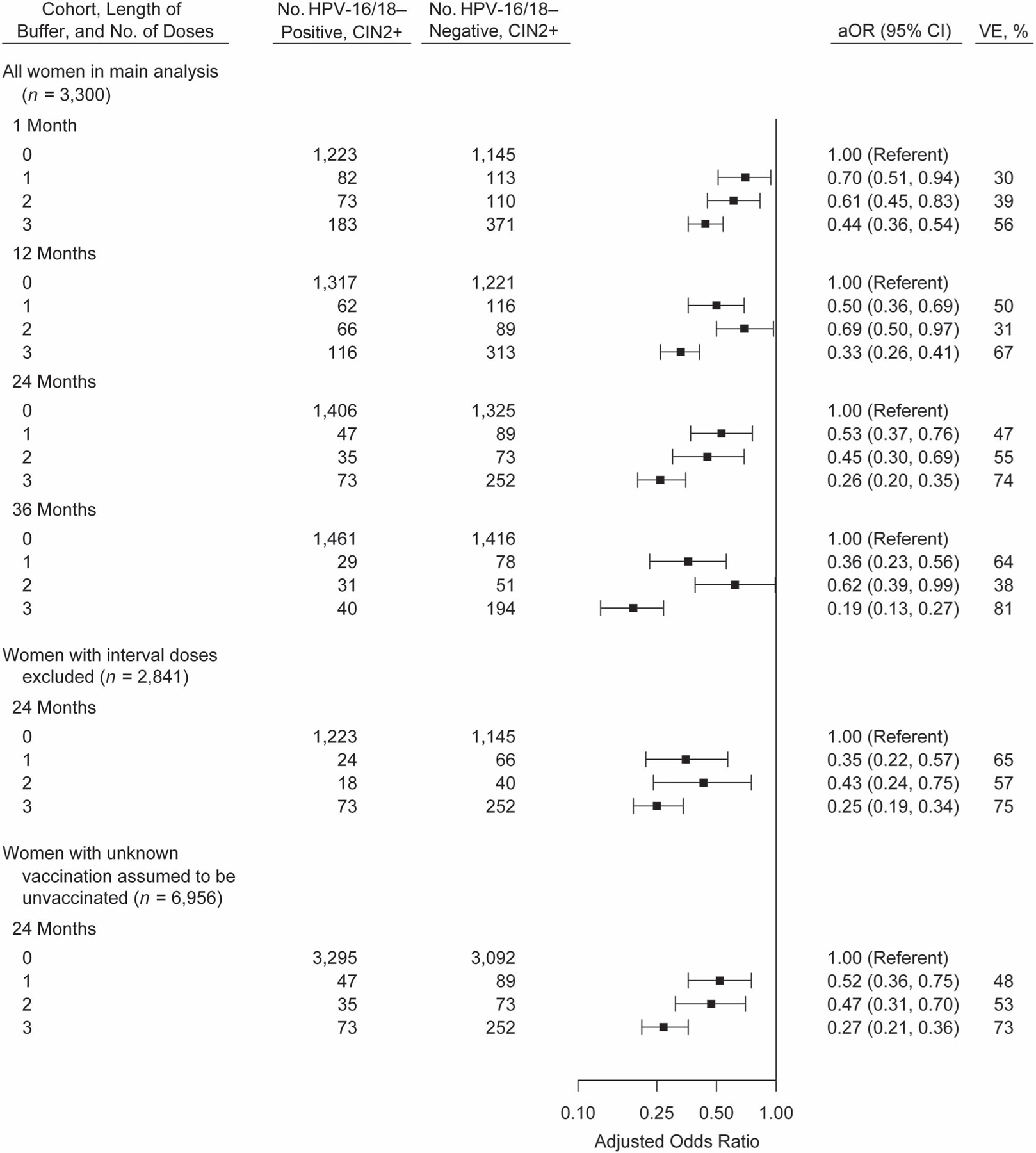
Vaccination history of women with cervical intraepithelial neoplasia grades 2–3 and adenocarcinoma in situ (CIN2+) with and without human papillomavirus (HPV) type 16 or 18 (HPV-16/18), adjusted odds ratios (aORs), and vaccine effectiveness (VE) in sensitivity analyses with varying classification of vaccine doses, HPV-IMPACT, United States, 2008–2014. Odds ratios were adjusted for surveillance site, race/ethnicity, and health insurance status. The buffer was defined as the time between the date of the first vaccine dose and the date of the screening test that led to CIN2+ diagnosis. Bars, 95% confidence intervals (CIs). HPV-IMPACT, Human Papillomavirus Vaccine Impact Monitoring Project.
