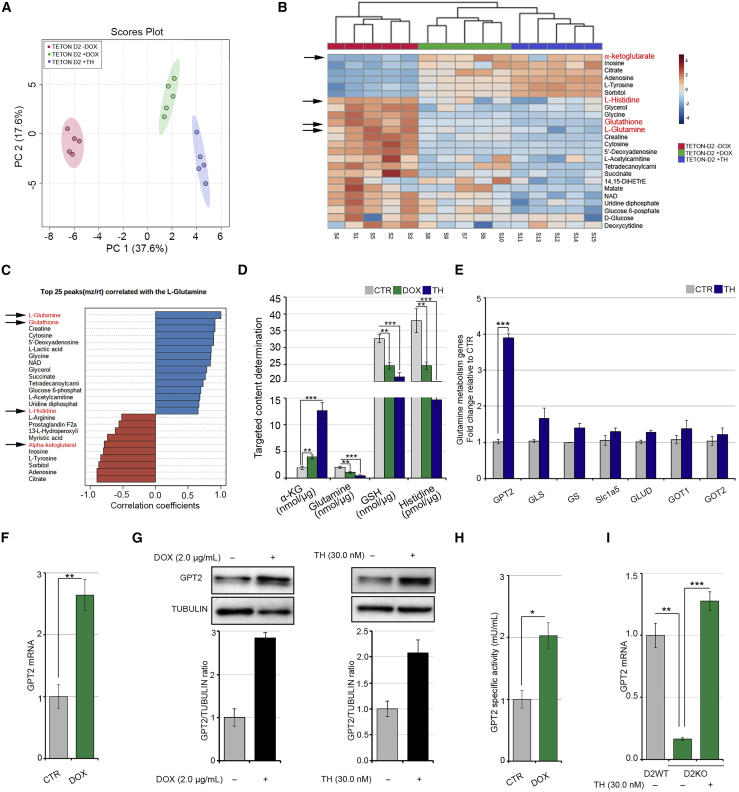
Figure 1.
Thyroid hormone induces metabolic changes in muscle cells by enhancing glutamine metabolism
(A) Principal-component analysis for metabolites profiled by GC/MS and extracted from C2C12 pTRE-D2 cells treated for 48 h with 2 μg/mL DOX, THs, and in untreated control (CTR) cells. The experiments were performed on five different biological replicates that were run in technical triplicates.
(B) Heatmap analysis for metabolites profiled by GC/MS as in (A). The rows show metabolites, and the columns represent the samples, p < 0.001.
(C) The top 25 peaks correlated with glutamine from the same analysis as in (A).
(B and C) Black arrows indicate the key metabolites involved in glutamine metabolism.
(D) Levels of α-KG, glutamine, GSH, and histidine determined by targeted GC-MS analysis in the same cells as in (A).
(E) mRNA expression levels of a panel of genes involved in glutamine metabolism were measured by real-time PCR in C2C12 cells cultured with or without THs for 24 h.
(F) mRNA expression levels of the GPT2 gene in C2C12 pTRE-D2 cells cultured with or without 2 μg/mL DOX for 48 h.
(G) Protein levels of GPT2 were measured by western blot analysis in C2C12 pTRE-D2 cells treated or not with 2 μg/mL DOX (left) and in C2C12 cells treated or not with THs (right) for 48 h. The histograms below show the quantification of the GPT2 protein levels versus tubulin levels.
(H) Enzymatic activity of GPT2 was measured in C2C12 pTRE-D2 cells treated or not with 2 μg/mL DOX for 48 h.
(I) mRNA expression levels of GPT2 gene were measured in D2 wild-type (D2WT) muscle stem cells and in D2-knock out (D2KO) muscle stem cells treated or not with THs for 48 h. Cyclophilin-A served as an internal CTR. Normalized copies of the indicated genes in CTR cells were set as 1. Data represent the mean ± standard deviation of the mean of fifteen replicates. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.