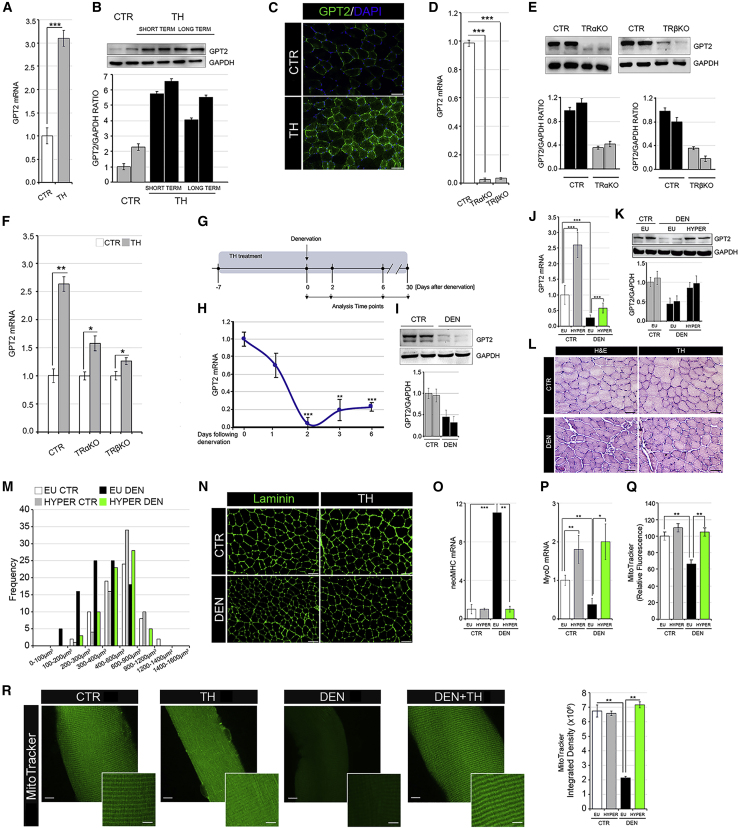
Figure 4.
Thyroid hormone promotes GPT2 expression in in vivo muscles, ameliorating muscle response to denervation
(A and B) Real-time PCR (A) and western blot (B) analyses of GPT2 in GC muscles of euthyroid (CTR) and hyperthyroid mice (short and long term).
(C) Immunofluorescence of GPT2 protein in CTR and hyperthyroid (TH) tibial anterior (TA) muscles. Scale bar, 50 μm.
(D) Basal mRNA expression levels of the GPT2 gene were measured in GC muscles collected by WT (CTR), TrαKO, and TRβKO mice.
(E) Protein levels of GPT2 were measured in the same muscles as in (D). Relative quantification of the GPT2 protein levels versus GAPDH levels is represented by histograms below.
(F) GPT2 mRNA levels were measured by real-time PCR in euthyroid and hyperthyroid CTR, TRαKO, and TRβKO GC muscles.
(G) Time-line of TH treatment and denervation experiments.
(H) mRNA expression levels of GPT2 gene were measured in innervated (CTR) and denervated (DEN) GC muscles after 1, 2, 3, and 6 days of sciatic nerve cut.
(I) Western blot analysis of GPT2 in GC CTR muscles and in GC muscles 6 days following denervation (DEN). GAPDH was used as a loading CTR.
(J) mRNA expression levels of GPT2 gene were measured in non-denervated (CTR) and denervated (DEN) GC muscles from euthyroid or hyperthyroid mice (EU and HYPER) 6 days following denervation.
(K) Western blot analysis of GPT2 in non-denervated (CTR) and denervated (DEN) GC muscles from euthyroid or hyperthyroid mice (EU and HYPER) after 6 days of sciatic nerve cut. Histograms below show the quantification of the GPT2 protein levels versus GAPDH levels.
(L and M) H&E staining (L) and cross-sectional area (CSA) (M) of TA muscles as in (J). Scale bar, 50 μm.
(N) Immunofluorescence of laminin in the same TA muscles as in (J). Scale bar, 50 μm.
(O and P) mRNA expression levels of neoMHC (O) and MyoD (P) were measured in the same GC muscles as in (J). Levels of indicated genes are relative to Cyclophilin-A mRNA used as an internal CTR and normalized to the CTR euthyroid muscle, set as 1.
(Q) Mitochondrial activity was measured by fluorescence-activated cell sorting (FACS) using MitoTracker Red CMXRos dye in non-denervated (CTR) and denervated (DEN) GC muscles from euthyroid or hyperthyroid mice (EU and HYPER) 2 days following denervation. The bar graph shows the relative median fluorescence versus CTR muscles arbitrarily set at 100.
(R) Confocal images of MitoTracker-stained extensor digitorum longus (EDL) muscle fibers derived from same mice as in (Q). Data are presented as overviews (top rows) and higher magnifications (bottom rows). Scale bars: 10 μm (top rows) and 5 μm (bottom rows). The histogram (right) shows the quantification of MitoTracker staining of muscle fibers. Data represent the mean ± SD of the mean of nine replicates. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.