Figure 3.
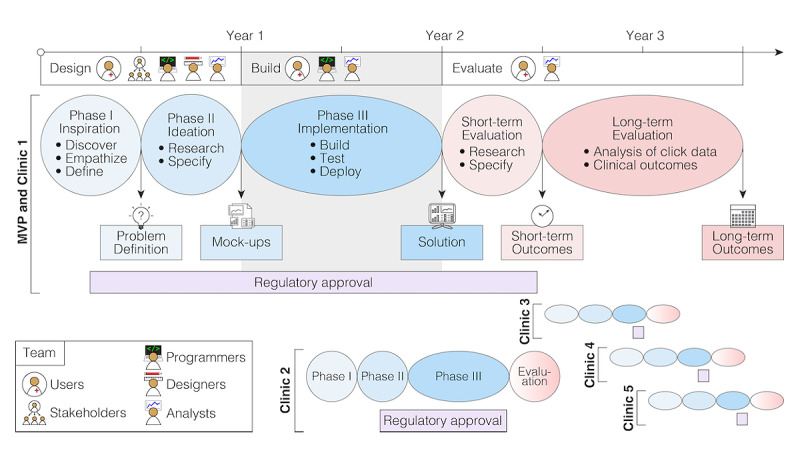
Example timelines and milestones of clinical application development. The design and development of an institution-specific platform for clinical applications, such as BRIDGE, is a multiyear undertaking (eg, 2 years for the MVP and clinic 1). Following the principles and phases of human-centered design ensures the development of a product that meets the needs of the users and the requirements of the institution. Obtaining institutional regulatory approval—a process that runs in parallel with the design and development processes—is critical and can risk becoming the rate-limiting step. The evaluation of the product initially focuses on user experience, followed by clinical outcomes such as morbidity, mortality, or efficiency. With the majority of the platform built, the design and build times are dramatically reduced for clinic 2, and they continue to fall as the process becomes refined (eg, under 6 months for clinics 3-5) and occurs in parallel. MVP: minimum viable product.
