Figure 4.
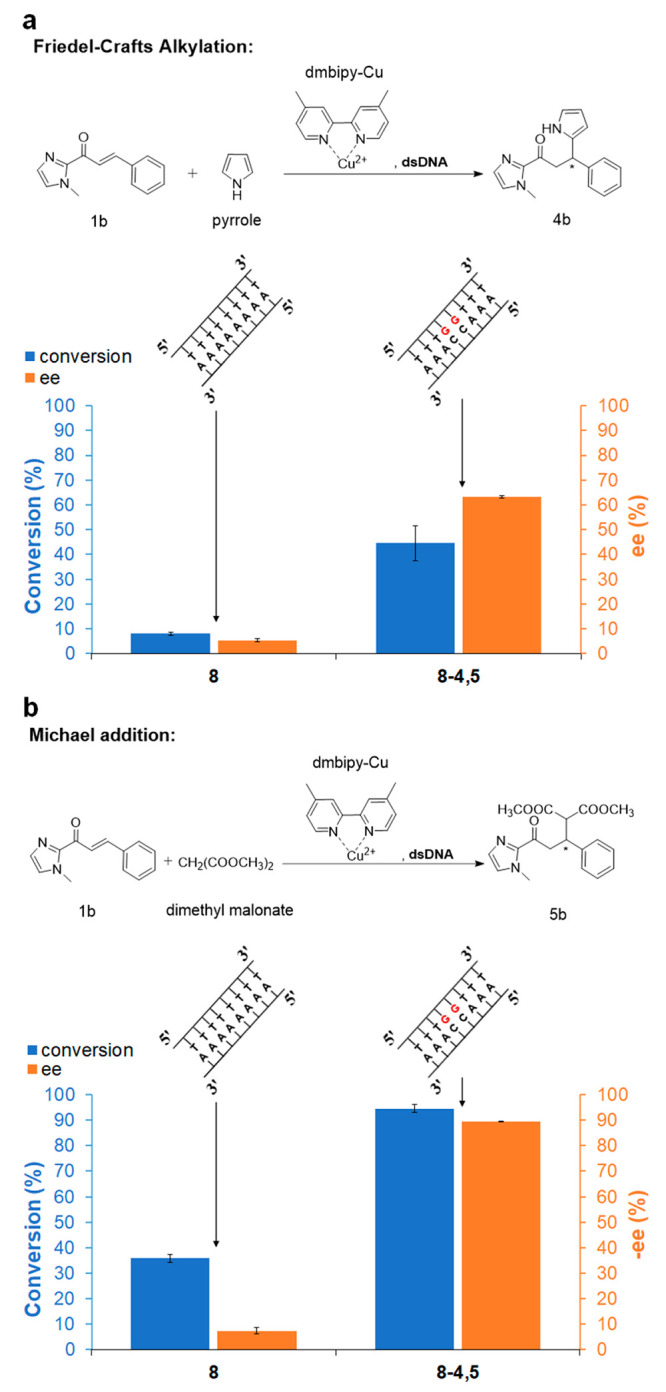
(a) Conversion and ee for Friedel–Crafts alkylation catalyzed by “8” or “8-4,5” with dmbipy-Cu. ee% = (moles of R enantiomer – moles of S enantiomer)/(moles of R enantiomer + moles of S enantiomer) × 100%.15 (b) Conversion and −ee for Michael addition reaction catalyzed by “8” or “8-4,5” with dmbipy-Cu. ee is negative for this reaction. −ee% = (moles of R enantiomer – moles of S enantiomer)/(moles of R enantiomer + moles of S enantiomer) × 100%.20 All reactions were carried out in MOPS (20 mM, pH 6.5) at 4 °C for 7 days; synthetic dsDNA: 50 μM; [dmbipy-Cu]: 50 μM; α,β-unsaturated 2-acyl imidazole (1b): 1 mM (i.e., 5% catalyst loading); pyrrole: 5 mM; dimethyl malonate: 11.4 μL (100 equiv). See Experimental Section (SI) for reaction procedure details. All data are averaged over three independent experiments. Parameters were determined for all displayed products by HPLC analysis on a chiral stationary phase.
