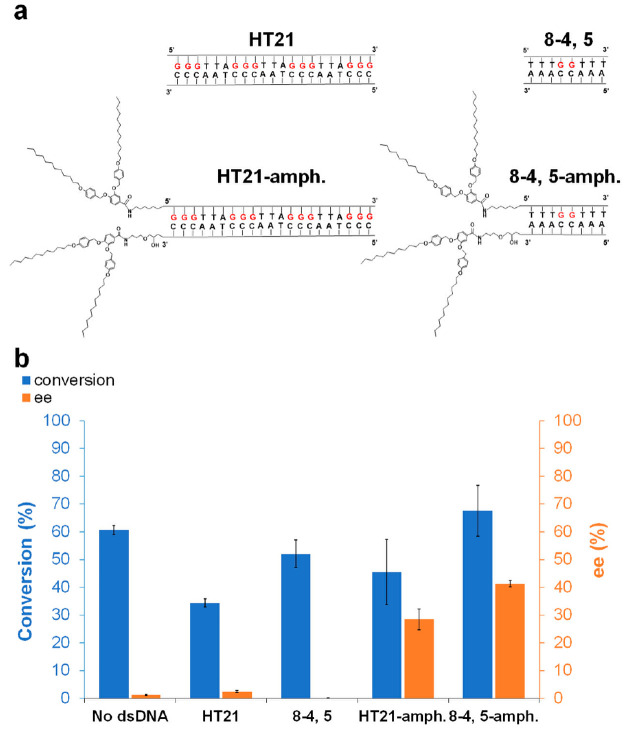
Figure 5.
(a) Chemical structures of “HT21”, “8-4,5”, “HT21-amph.”, and “8-4,5-amph.” (b) Conversion and ee of Diels–Alder reaction catalyzed by different dsDNA sequences with dmbipy-Cu in methanol–water mixture (v/v = 50/50). All reactions were carried out in MOPS (20 mM, pH 6.5) at 4 °C for 48 h, dsDNA: 15 μM; [dmbipy-Cu]: 15 μM; aza chalcone (1a): 1 mM (i.e., 1.5% catalyst loading); cyclopentadiene: 5.6 μL (67 equiv). See Experimental Section (SI) for reaction procedure details. All data are averaged over three independent experiments. Parameters were determined for all displayed products by HPLC analysis on a chiral stationary phase.