Table 2.
Hydroamination of 1,3-Diene (2a) with Various Azoles[a]
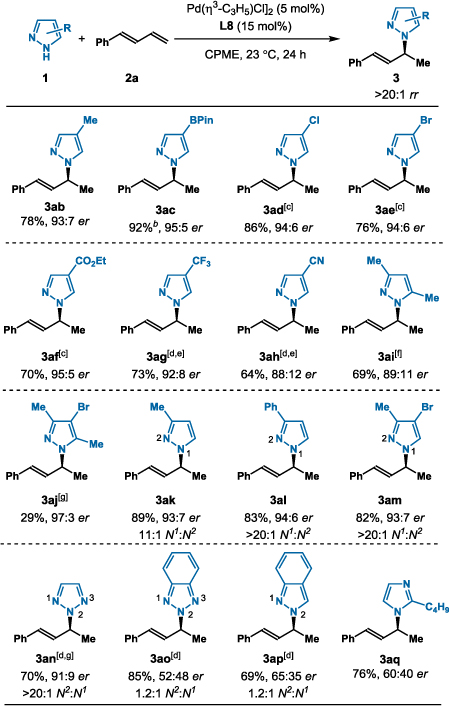
|
Reaction conditions: 1 (0.2 mmol), 2a (1.0 mmol), [Pd(η3-C3H5)Cl]2 (5 mol%), MeO-BIPHEP L8 (15 mol%), CPME (0.8 mL), 23 °C, 24 h. Isolated yields. Regioselectivity determined by 1H NMR analysis of the unpurified reaction mixture. Enantioselectivity determined by chiral SFC.
NMR yield.
48 h
60 °C
12 h
8 h
36 h.
