Fig. 4.
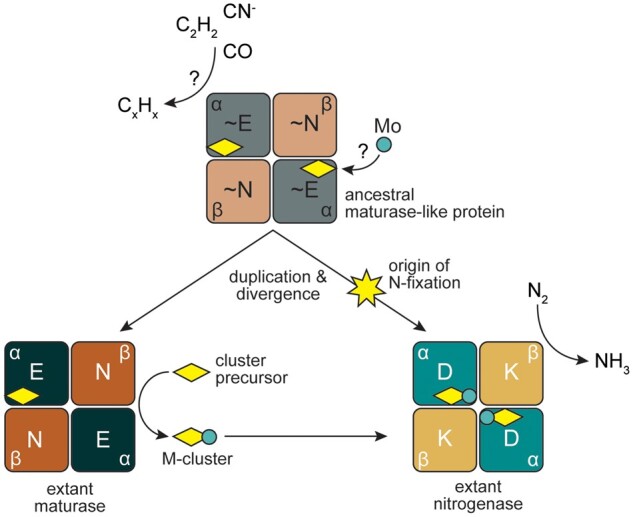
Proposed model for the origins and functional divergence of maturase and nitrogenase proteins. An ancestral maturase-like protein (∼NifEN, gray and light brown), incapable of reducing N2, may have otherwise reduced various carbon-containing substrates and/or played a role in cluster (yellow diamond) biosynthesis. The ancestor may have been capable of incorporating molybdenum (teal circle) into the cluster. Duplication of the encoding ancestral genes and functional divergence would then have yielded canonical maturase (NifEN, dark green and brown) and nitrogenase (NifDK, teal and yellow) proteins. Maturases would have specialized to provide a scaffold for the maturation of the nitrogenase cluster precursor (yellow diamond) to the nitrogenase active-site M-cluster (yellow diamond with teal circle). In parallel, tuning of the ancestral peptide environment along a divergent lineage would have spurred the origin of N2 reduction and specialization of nitrogenases for a solely catalytic role in nitrogen fixation. Protein components of the α2β2 heterotetrameric nitrogenase and maturase structures are labeled.
