Introduction
In patients with acute coronary syndrome (ACS), dual antiplatelet therapy (DAPT) with ticagrelor 90 mg twice daily (b.i.d.) on top of acetylsalicylic acid (ASA) is recommended for 12 months to reduce adverse thrombotic events. In subjects at high ischemic risk who have tolerated DAPT without a bleeding complication, continuation of ticagrelor 60 mg b.i.d. for longer than 12 months may be considered [1].
Increased ischemic risk occurs in the early period after ACS, with elevated rates of clinical events clustering during the first month, which is reflective of elevated platelet reactivity [2]. On the other hand, bleeding risk increases in a stepwise fashion after cumulative administration of an antiplatelet agent. It is related to the duration and dose of the antiplatelet treatment, and the majority of bleeding events occur after 30 days following ACS [2]. This means that the ischemic component should be targeted with potent antiplatelet strategies in the earliest phase after ACS, whereas de-escalation of the antiplatelet therapy could be justified after clinical stabilization occurs.
Pharmacodynamic data show that a reduction of ticagrelor bioavailability by ~30% significantly decreases its antiplatelet effect in patients with acute myocardial infarction (MI), but not in stable subjects with prior MI [3, 4]. Still, in patients > 1 year after MI the equivalent pharmacodynamic effects of ticagrelor 90 mg b.i.d. and 60 mg b.i.d. provide comparable clinical efficacy, with a better tolerability of treatment observed with a lower dose [3, 5]. Recently, it was demonstrated that ticagrelor 60 mg b.i.d. also provides a similar antiplatelet effect to 90 mg b.i.d. already 1 month after MI [6].
In the TWILIGHT study, high-risk patients who had undergone percutaneous coronary intervention (PCI) and were treated with ticagrelor 90 mg b.i.d. monotherapy following 3 months of standard DAPT, experienced fewer bleeding events than patients receiving ticagrelor with ASA, without ischemic harm over a period of 1 year [7].
It was hypothesized herein, that the reduction of ticagrelor maintenance dose to 60 mg b.i.d. 1 month after ACS, followed by ASA withdrawal at 3 months after ACS will result in improved safety and tolerability of treatment with preserved anti-ischemic benefit [8]. The aim of the Evaluation of safety and efficacy of two ticagrelor-based de-escalation antiplatelet strategies in acute coronary syndrome — a randomized clinical trial (ELECTRA-SIRIO 2) is to assess the influence of early ticagrelor dose reduction with or without discontinuation of ASA on clinically relevant bleeding and maintenance of anti-ischemic efficacy after ACS.
Methods
Study design and population
The ELECTRA-SIRIO 2 study is a phase III, randomized, multicenter, double-blind, investigator-initiated clinical trial with a 12 month follow-up. The study population will include 4500 patients admitted to the study centers due to ACS, including ST-segment elevation myocardial infarction (STEMI), non-ST-segment elevation myocardial infarction (NSTEMI) and unstable angina (UA). The diagnosis of STEMI and NSTEMI will be made according to the Fourth Universal Definition of Myocardial Infarction [9], and UA will be diagnosed according to the 2020 European Society of Cardiology (ESC) guidelines for the management of non-ST-segment elevation ACS (NSTE-ACS) [10]. STEMI patients will have to be qualified for the primary PCI to be eligible for the inclusion. To be enrolled into the study, patients with NSTE-ACS (NSTEMI or UA) will have to fulfill at least one of the following criteria: 1) ≥ 60 years old; 2) previous MI or coronary artery by-pass grafting; 3) ≥ 50% stenosis in ≥ 2 coronary arteries; 4) previous ischemic stroke or transient ischaemic attack; 5) ≥ 50% carotid stenosis or cerebral revascularization; 6) diabetes mellitus; 7) peripheral artery disease; 8) chronic kidney disease with glomerular filtration rate (GFR) < 60 mL/min. The exclusion criteria include, among others, indications for oral anticoagulation therapy and end stage kidney disease with GFR < 15 mL/min or on dialysis. Supplementary Appendix contains the complete list of inclusion and exclusion criteria.
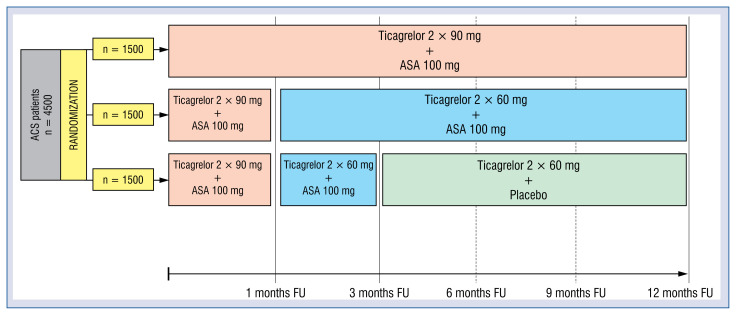
All participants will receive loading doses of 180 mg ticagrelor and 300 mg ASA. Patients loaded with clopidogrel before the study inclusion will be re-loaded with 180 mg ticagrelor upon enrolment. Participants will be randomized in a 1:1:1 ratio into the following arms: low-dose ticagrelor with ASA (LDTA), low-dose ticagrelor with placebo (LDTP), and standard-dose ticagrelor with ASA (SDTA), the latter being the control arm. During the first month after ACS patients from all three groups will receive a standard DAPT with ticagrelor 90 mg b.i.d and 100 mg ASA once daily. Patients assigned to the control group (SDTA) will continue this treatment for 12 months. Patients allocated to the experimental arms (LDTA and LDTP) will receive reduced maintenance dose of ticagrelor 60 mg b.i.d. and 100 mg ASA q.d starting after the first month following ACS. Patients from the LDTA arm will continue this treatment until 12 months after ACS, while patients from the LDTP arm will additionally discontinue ASA 3 months after ACS and continue on ticagrelor 60 mg b.i.d. monotherapy until 12 months after ACS. All participants are expected to undergo 5 out-patient follow-up visits as depicted in Figure 1.
Figure 1.
Design of the trial; ASA — acetylsalicylic acid; ACS — acute coronary syndrome; FU — follow-up.
All study participants will be provided with blinded packages containing the antiplatelet medications (ticagrelor 60 mg or 90 mg, and ASA 100 mg or placebo) according to the randomized allocation. The dispended medications will be free of charge and will be sufficient to cover the whole period (12 months) of each patient in the study.
Each patient will provide written informed consent to participate in the study. The study will be conducted in accordance with the Good Clinical Practice guidelines and with the regulations contained in the Declaration of Helsinki. The trial was approved by the appropriate Ethics Committee to conduct the study (study approval reference number KB 379/2020). ClinicalTrials.gov Identifier: NCT04718025.
Treatment protocol and concomitant medications
Apart from the investigated strategies, enrolled patients will be treated according to the current ESC guidelines, however cholesterol-lowering treatment with high doses of statins will only be allowed (≥ 40 mg atorvastatin or ≥ 20 mg rosuvastatin), unless contraindicated, and the addition of ezetimibe will be recommended. Use of stents with ultra-thin or thin struts will be highly recommended during PCI in order to decrease the thrombotic risk related to stent implantation [11].
Study endpoints
The primary safety composite endpoint is the first occurrence of type 2, 3 or 5 bleeding according to the Bleeding Academic Research Consortium (BARC) criteria. The primary efficacy endpoint is the composite of time to death from any cause, first nonfatal MI, or first nonfatal stroke. The key secondary endpoint, net clinical effect, was defined as composite of death from any cause, nonfatal MI, or nonfatal stroke, and the first occurrence of BARC type 2, 3, or 5 bleeding. Remaining secondary endpoints include: death from any cause, cardiovascular death, MI, ischemic stroke, definite or probable stent thrombosis, dyspnea, BARC type 3 or 5 bleeding, Thrombolysis in Myocardial Infarction (TIMI) major or minor bleeding, Global Use of Strategies to Open Occluded Coronary Arteries (GUSTO) moderate or severe or life-threatening bleeding, International Society on Thrombosis and Hemostasis (ISTH) major bleeding.
Sub-group analyses
Prespecified subanalyses will be performed according to: 1) diabetes mellitus; 2) chronic kidney disease (GFR < 60 mL/min/1.73 m2); 3) gender; 4) age; 5) type of ACS; 6) administration of morphine during the index event; 7) presence or absence of multivessel disease. Additionally, impact of the following characteristics on the clinical outcomes will be evaluated: 1) complexity of coronary revascularization; 2) lipid-lowering treatment; 3) results in the MEDMOTION project (Suppl. Appendix).
Safety monitoring
The trial will be overseen by the international Steering Committee, clinical events committee, and data and safety monitoring board (DSMB). The safety of the tested antiplatelet strategies, all clinical events, and any deviations to the study protocol will be periodically monitored based on electronic medical documentation by an independent DSMB. Based on the safety data, the DSMB may recommend modifications to the protocol, suspension or termination of the study. All final decisions regarding trial modifications rest with the Steering Committee.
Statistical analysis
Sample size and power calculation were based on a superiority assumption for the primary safety end-point for LDTP vs. SDTA arm. Assuming a bleeding incidence of 7.1% at 1 year with standard dose ticagrelor plus ASA (rate reported in the TWILIGHT study [7]), a sample size of 1178 patients per arm is required to provide 95% power to detect 40% lower incidence of the primary safety composite endpoint in LDTP vs. SDTA group (43.6% relative reduction observed in ticagrelor monotherapy arm of the TWILIGHT trial), with a type I error rate of 0.05.
The primary efficacy endpoint (composite of death from any cause, nonfatal MI, or nonfatal stroke) will be evaluated with the use of a prespecified noninferiority hypothesis (LDTP vs. SDTA). Under the assumption of an incidence of 10.2% (occurrence rate reported for this endpoint in the PLATO study at 1 year in the SDTA, a sample size of 1204 patients per arm is needed to provide 90% power to rule out an absolute difference in risk of 1.6 percentage points, with a one-sided type I error rate of 0.025 (assumption made for the sample size calculations made in the TWILIGHT study).
Enrolment of a total of 4500 patients (1500 in each arm) is planned to compensate the potential drop-out from the study up to 20%. This broad margin has been chosen as the time between randomization and actual onset of the investigated strategies is 1 and 3 months for experimental LDTA and LDTP strategies, respectively, which may increase the risk of drop-out before the beginning of the allocated regimen.
Discussion
The primary hypothesis of the ELECTRA-SIRIO 2 study is that monotherapy with low-dose ticagrelor in ACS patients will lead to a significant reduction of clinically relevant bleeding compared with standard-dose ticagrelor with ASA. The additional study arm including DAPT with low-dose ticagrelor is intended to differentiate the impact of decreasing the ticagrelor dosage versus eliminating ASA from the antiplatelet treatment.
During the first month after ACS, increased platelet reactivity goes in pair with increased rate of adverse ischemic events. Therefore, DAPT with ticagrelor 90 mg b.i.d. is necessary to obtain adequate platelet inhibition and prevent thrombotic events during the initial phase of ACS treatment. Occurrence of thrombotic complications decreases over time, and reaches a stable level approximately 1 month after ACS which is related to reduced baseline platelet activation and potentially may allow treatment de-escalation [2].
A sub-study of the PEGASUS-TIMI 54 trial showed that in stable patients > 1 year after MI, ticagrelor 60 mg b.i.d. provides similar platelet inhibition as 90 mg b.i.d., explaining comparable clinical efficacy of both doses in this setting [3, 5]. Recently, it was reported that the same pharmacodynamic effects of low-dose vs. standard-dose ticagrelor already after 1 month following ACS. In the ELECTRA pilot study, there were no differences between the two regimens with regard to on-ticagrelor platelet inhibition, and the number of patients with optimal platelet reactivity was identical between the arms [6].
On the other hand, antiplatelet treatment is burdened with non-negligible side effects, greatly related to bleeding, that often may require medical attention or lead to discontinuation of treatment (e.g., rate of premature discontinuation of antiplatelet treatment in the PLATO study was 22–23%) [2]. Premature discontinuation of antiplatelet therapy, especially in invasively-treated patients, may lead to detrimental cardiovascular and thrombotic events, such as recurrent ACS or stent thrombosis. Several strategies aiming to enhance safety of antiplatelet treatment, without reducing its efficacy, have been evaluated so far.
Platelet function testing-guided de-escalation from prasugrel to clopidogrel was shown to be non-inferior to standard treatment with prasugrel at 1 year after PCI in terms of net clinical benefit. However, with this approach 39% of patients required a switch-back to prasugrel due to commonly observed insufficient platelet inhibition [12]. In another study, DAPT downgrading from prasugrel/ticagrelor to clopidogrel 1 month after ACS was associated with a net clinical benefit driven by a reduction in bleeding complications, with unchanged risk of recurrent ischemic events [13]. Nonetheless, the SCOPE registry reported switching from novel P2Y12 receptor inhibitors to clopidogrel as an independent predictor of net adverse cerebrovascular events [14].
An interesting approach to decrease bleeding complications was evaluated in the TWILIGHT study. This trial has shown that switching from DAPT with ticagrelor 90 mg b.i.d. and ASA to ticagrelor 90 mg b.i.d. monotherapy at 3 months after ACS leads to a significant reduction in bleeding, with maintained antithrombotic effectiveness [7].
The antiplatelet de-escalation strategies proposed in the current trial (LDTA and LDTP) are expected to essentially decrease the incidence of clinically significant bleeding events during the first year after ACS without increasing the rates of thrombotic events. In contrast to the platelet function testing-guided de-escalation strategies, the concept proposed in the ELECTRA-SIRIO 2 study does not require platelet reactivity assessment, making this step-down approach more feasible for wide application in clinical practice [8]. Discontinuation of ASA, as investigated in the TWILIGHT study, resulted in reduction in clinically relevant bleeding episodes, including fatal bleeds, by 43% [7]. It can be assumed that lowering the daily dose of ticagrelor may only further decrease the rate of bleeding episodes.
Additionally, due to the expected dose-dependent reduction in therapy-related adverse effects, including dyspnea or bradycardia, an improved adherence to the treatment may be anticipated. In the PEGASUS-TIMI 54 trial dyspnea occurred less frequently in patients who received the lower dose of ticagrelor compared with those treated with the standard dose (16% vs. 19%) [5]. This also might be of importance as early termination of ticagrelor leaves ACS patients unprotected against ischemic consequences.
The TWILIGHT trial proved that monotherapy with a standard ticagrelor dose in high-risk stable patients is safer, but still equally effective, compared with ticagrelor-based DAPT. On the other hand, the ELECTRA pilot study showed the same level of platelet inhibition with standard and reduced ticagrelor doses in stable patients already 1 month after PCI for ACS [6]. To date, deescalation of antiplatelet therapy in ACS patients based on decreasing the dose of ticagrelor with or without discontinuation of ASA has never been tested in a large randomized clinical trial. The design of the ELECTRA-SIRIO 2 trial includes both these strategies aiming to document reduction of clinically relevant bleeding, without compromising clinical efficacy in terms of prevention of adverse cardiovascular events.
Supplementary Information
Footnotes
Funding
This research received financial support from the Medical Research Agency, Poland, through Project no. 2019/ABM/01/00009.
Conflict of interest: Jacek Kubica received lecture honoraria from AstraZeneca; Diana Gorog received lecture honoraria from AstraZeneca and Boehringer Ingelheim, and a grant from Bayer; Aldona Kubica received lecture honoraria from AstraZeneca; Andrzej Budaj received investigator’s fee, advisory board and lecture honoraria from AstraZeneca, Bayer, Bristol Myers Squibb, GlaxoSmithKline, Sanofi Aventis, investigator’s fee and lecture honoraria from Novartis, and investigator’s fee from Eisai and Amgen; Jolanta Siller-Matula received personal fees from Bayer, BMS, Chiesi, and Daiichi; Paul A. Gurbel received grants and personal fees from Bayer HealthCare LLC, Otitopic Inc, Amgen, Janssen, and US WorldMeds LLC, and grants from Instrumentation Laboratory, Haemonetics, Medicure Inc., Idorsia Pharmaceuticals, and Hikari Dx.; Dimitrios Alexopoulos received personal fees from AstraZeneca; Jolita Badarienė received investigator’s fee and lecture honoraria from AstraZeneca, investigator’s fee from Amgen, and lecture honoraria from Bayer; Evangelos Giannitsis received lecture honoraria, research funding and consultancy fees from Roche Diagnostics, lecture honoraria and research funding Daiichi Sankyo, lecture honoraria and consultancy fees from AstraZeneca, Boehringer Ingelheim, Bayer Vital, and BRAHMS GmbH, and consultancy fees from Indorsia, Radiometer, and Hoffmann-La Roche; Miłosz Jaguszewski received lecture honoraria from AstraZeneca; Stefan James received grants and speaker fees from AstraZeneca, Eli Lilly, Bayer, Jansen, and The MedCo.; Young-Hoon Jeong received personal fees from AstraZeneca, Sanofi-Aventis, and Daiichi Sankyo, personal fees and grants from Han-mi Pharmaceuticals and Yuhan Pharmaceuticals, grants from U&I Corporation, and non-financial support from Haemonetics; Udaya Tantry received honoraria from UptoDate; Wojciech Wojakowski received lecture honoraria from AstraZeneca; Jarosław Kasprzak received lecture honoraria from Astra-Zeneca and Bayer; Jacek Legutko received advisory board and lecture honoraria from AstraZeneca and Gedeon Richter; Maciej Lesiak received speaker and consultancy honoraria from AstraZeneca and Bayer; Eliano P. Navarese received grants from Abbott, Amgen, and lecture honoraria from Amgen, Astra-Zeneca, Bayer, Pfizer and Sanofi-Regeneron. All other authors have nothing to disclose.
References
- 1.Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87–165. doi: 10.1093/eurheartj/ehy394. [DOI] [PubMed] [Google Scholar]
- 2.Wallentin L, Becker R, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045–1057. doi: 10.1056/nejmoa0904327.. [DOI] [PubMed] [Google Scholar]
- 3.Storey RF, Angiolillo DJ, Bonaca MP, et al. Platelet inhibition with ticagrelor 60 mg versus 90 mg twice daily in the PEGASUS-TIMI 54 trial. J Am Coll Cardiol. 2016;67(10):1145–1154. doi: 10.1016/j.jacc.2015.12.062. [DOI] [PubMed] [Google Scholar]
- 4.Kubica J, Adamski P, Ostrowska M, et al. Morphine delays and attenuates ticagrelor exposure and action in patients with myocardial infarction: the randomized, double-blind, placebo-controlled IMPRESSION trial. Eur Heart J. 2015;37(3):245–252. doi: 10.1093/eurheartj/ehv547.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonaca MP, Bhatt DL, Cohen M, et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372(19):1791–1800. doi: 10.1056/NEJMoa1500857. [DOI] [PubMed] [Google Scholar]
- 6.Kubica J, Adamski P, Buszko K, et al. Platelet inhibition with standard vs. lower maintenance dose of ticagrelor early after myocardial infarction (ELECTRA): a randomized, open-label, active-controlled pharmacodynamic and pharmacokinetic study. Eur Heart J Cardiovasc Pharmacother. 2019;5(3):139–148. doi: 10.1093/ehjcvp/pvz004. [DOI] [PubMed] [Google Scholar]
- 7.Mehran R, Baber U, Sharma S, et al. Ticagrelor with or without Aspirin in High-Risk Patients after PCI. N Engl J Med. 2019;381(21):2032–2042. doi: 10.1056/nejmoa1908419.. [DOI] [PubMed] [Google Scholar]
- 8.Kubica J, Adamski P, Niezgoda P, et al. A new approach to ticagrelor-based de-escalation of antiplatelet therapy after acute coronary syndrome. A rationale for a randomized, double-blind, placebo-controlled, investigator-initiated, multicenter clinical study. Cardiol J. 2021;28(4):607–614. doi: 10.5603/CJ.a2021.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction. Eur Heart J. 2019;40(3):237–269. doi: 10.1093/eurheartj/ehy462. [DOI] [PubMed] [Google Scholar]
- 10.Collet JP, Thiele H, Barbato E, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42(14):1289–1367. doi: 10.1093/eurheartj/ehaa575. [DOI] [PubMed] [Google Scholar]
- 11.Zaman A, de Winter RJ, Kogame N, et al. Safety and efficacy of a sirolimus-eluting coronary stent with ultra-thin strut for treatment of atherosclerotic lesions (TALENT): a prospective multicentre randomised controlled trial. Lancet. 2019;393(10175):987–997. doi: 10.1016/S0140-6736(18)32467-X. [DOI] [PubMed] [Google Scholar]
- 12.Sibbing D, Aradi D, Jacobshagen C, et al. Guided de-escalation of antiplatelet treatment in patients with acute coronary syndrome undergoing percutaneous coronary intervention (TROPICAL-ACS): a randomised, open-label, multicentre trial. Lancet. 2017;390(10104):1747–1757. doi: 10.1016/S0140-6736(17)32155-4. [DOI] [PubMed] [Google Scholar]
- 13.Cuisset T, Deharo P, Quilici J, et al. Benefit of switching dual antiplatelet therapy after acute coronary syndrome: the TOPIC (timing of platelet inhibition after acute coronary syndrome) randomized study. Eur Heart J. 2017;38(41):3070–3078. doi: 10.1093/eurheartj/ehx175.. [DOI] [PubMed] [Google Scholar]
- 14.De Luca L, D’Ascenzo F, Musumeci G, et al. Incidence and outcome of switching of oral platelet P2Y12 receptor inhibitors in patients with acute coronary syndromes undergoing percutaneous coronary intervention: the SCOPE registry. EuroIntervention. 2017;13(4):459–466. doi: 10.4244/EIJ-D-17-00092. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.