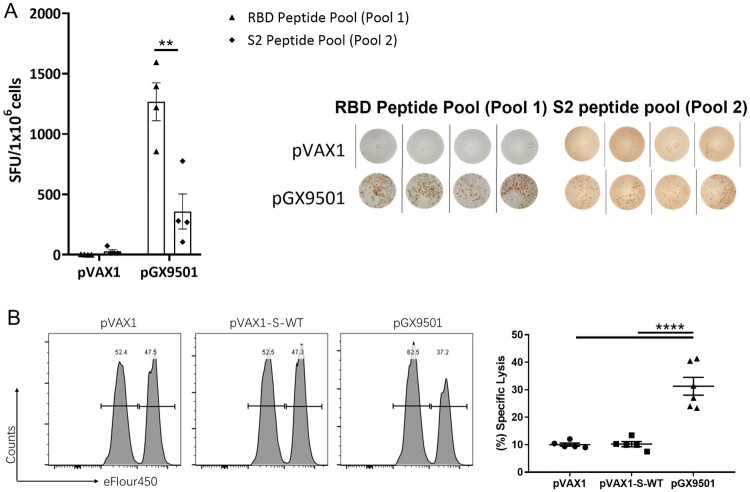
Figure 1.
Peptide pool 1 induced strong T cell responses in Balb/c mice. Balb/c mice (n = 5/group) were immunized twice two weeks apart with 25 μg pGX9501 or pVAX1 (empty vector). T cell responses were analyzed on day 14 after the second injection. (A) Splenocytes were harvested, and IFN-γ ELIspot T cell responses were measured after stimulation for 20 h with overlapping peptide pools 1 or 2. (B) Antigen-specific cytotoxic lymphocyte (CTL) killing activity was evaluated by an in vivo CTL assay. Target cells at 4 × 106/ml from naïve mice were peptide-pulsed with pool 1 then labelled with a high concentration of eFlour450 in vitro. Control cells were non-peptide-pulsed cells and labelled with a low concentration of eFluor450. The cells were mixed and transferred i.v. into immunized mice. After 5 h, splenocytes were harvested, and the intensity of eFlour450 peptide labelled target cells was compared with the non-peptide-labelled negative control cells by flow cytometry. pVAX1-s-WT was made from the wild type sequence of the full-length spike protein of the SARS-CoV-2(SARS-CoV-2/WH-09/human/2020/CHN) was subcloned into the pVAX1. The sequence of the same region was optimized via SynCon technology, synthesized, and cloned into pVAX1 as the pGX9501.