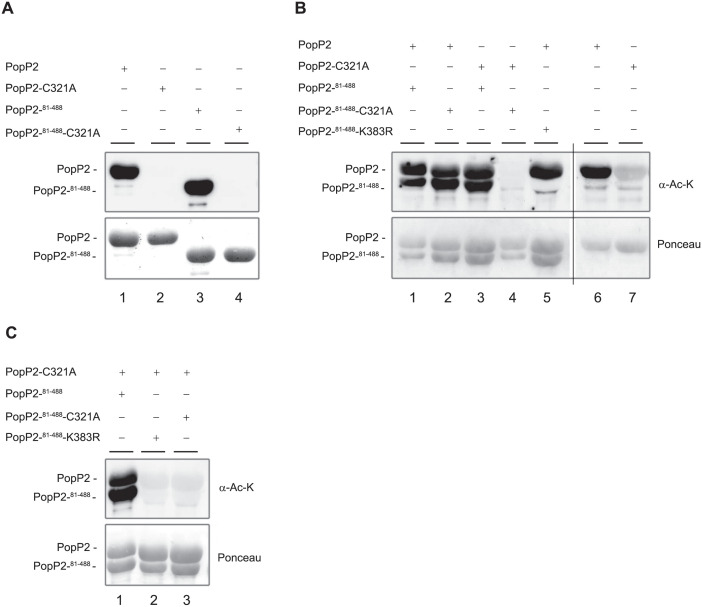
Fig 7. Intermolecular autoacetyl-transferase activity of PopP2 is dependent on the integrity of K383 residue.
A: A truncated form of PopP2 that lacks its first 80 amino acids (PopP2-81-488) retains its acetyltransferase activity. B: Detection of acetylated forms of GST-PopP2-C321A and GST-PopP2-C321A-81-488 upon co-expression with GST-PopP2-81-488 and GST-PopP2, respectively (lanes 2 and 3). K383R mutation prevents GST-PopP2 to acetylate GST-PopP2-81-488-K383R in trans (lane 5). The black line indicates image splicing (lanes 1 to 5 and lanes 6–7 are from the same experiment consisting in two membranes). C: Detection of acetylated forms of GST-PopP2-C321A upon co-expression with GST-PopP2-81-488 (lane 1) but not with GST-PopP2-C321A81-488 (lane 2) or GST-PopP2-81-488-K383R (lane 3). The indicated protein combinations were purified from E. coli and their acetylation status tested by immunoblot with an α-Ac-K antibody. The position of acetylated proteins is indicated by dashes (top). GST-purified PopP2 recombinant proteins are shown after Ponceau staining (bottom). A, B and C represent three independent experiments carried out under identical conditions.