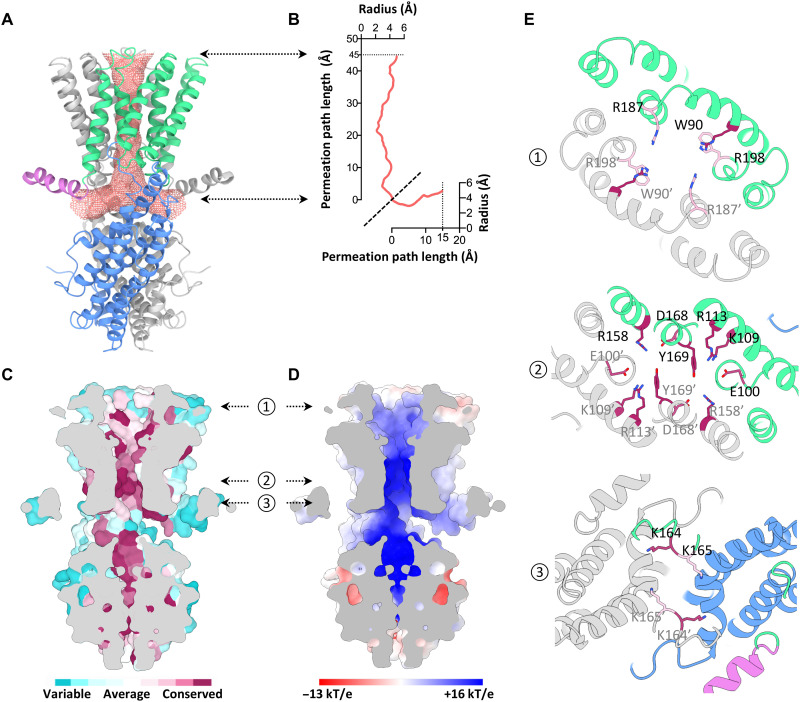
Fig. 3. Structural feature of the GmALMT12/QUAC1 channel pore.
(A) The pore-lining surface was computed by the program HOLE and drawn into a ribbon model of the GmALMT12/QUAC1 pore. We used a simple van der Waals surface for the protein and the program default probe radius of 1.15 Å. The T-shaped pore is shown in salmon dots. (B) Plot of the pore radius as a function of the pore axis. (C and D) Side views of the cross section through the ion-conducting pore. The molecular surface colored with sequence conservation for 137 nonredundant proteins of the QF2B subfamily is shown in (C), and the molecular surface colored with electrostatic potential is shown in (D). Electronegative and electropositive potential in (D) are colored in degrees of red and blue saturation, respectively. (E) Top views of the cross section through the ion-conducting pore at three indicated positions by the arrows in (C) and (D). The ribbon is colored as in (A). The conserved pore-lining charged residues are shown in sticks, colored with the sequence conservation as in (C).