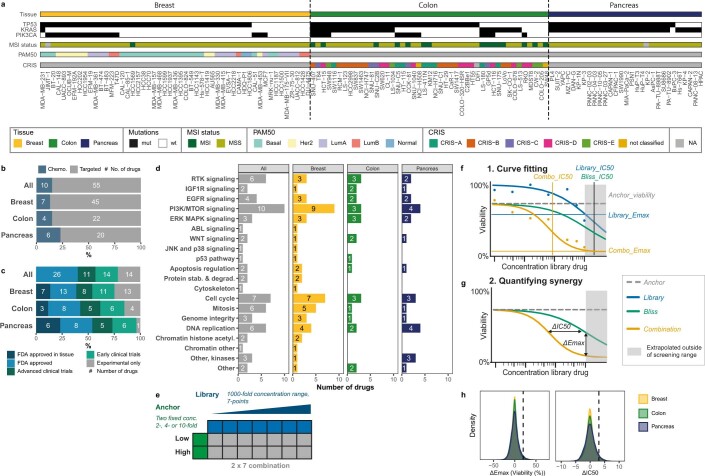
Extended Data Fig. 1. Information on cell lines, drugs and screen design.
a, OncoPrint detailing mutation status of three key mutations (TP53, KRAS, PIK3CA), MSI status, and clinical subtyping (PAM50 or CRIS where available) for all 125 cancer cell lines. b, Proportion of chemotherapeutic and targeted drugs screened. c, Proportion of drugs that are FDA-approved, in clinical trials, or are in development. d, Number of drugs screened per pathway and tissue. e, Schematic of anchored screening design. An anchor is tested at two fixed concentrations against a library screened at a 7-point, discontinuous 1,000-fold concentration range (two 2-fold dilution steps from the highest used concentration, all other dilution steps are 4-fold). f, Schematic of drug response curve fits of single-agent and combination responses. Vertical and horizontal lines are helper lines facilitating the reading of drug response metrics from the x-axis (concentration) and y-axis (viability), respectively. g, Schematic of synergy quantification based on efficacy (ΔEmax) or potency (ΔIC50). h, ΔEmax and ΔIC50 are normally distributed and only a minority meet synergy thresholds. Density distribution of ΔEmax (viability in %; left) and ΔIC50 (log2; right) across all combination responses. Vertical dashed lines represent synergy thresholds (ΔEmax ≥ 20% and ΔIC50 ≥ 3). n = 156,065 measurements in breast, n = 74,525 in colon, n = 66,117 in pancreas.