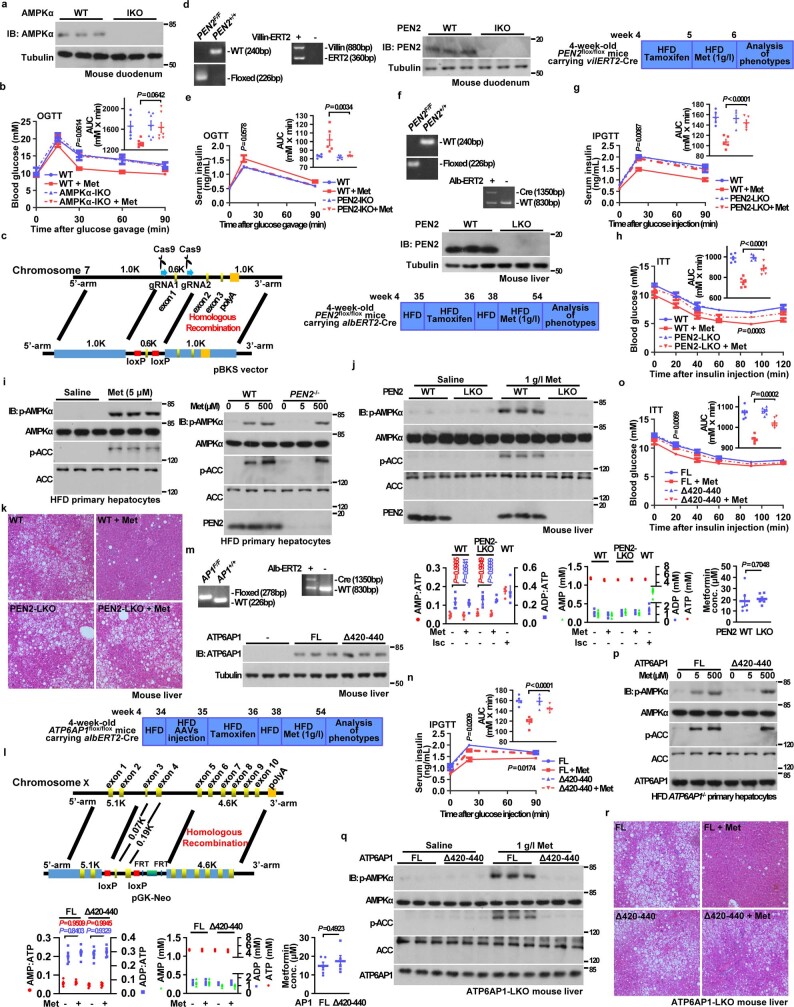
Extended Data Fig. 12. PEN2-ATP6AP1 axis is required for metformin-mediated glucose absorption and hepatic fat reduction.
a, Verification of AMPKα knockout efficiency in the duodenum of AMPKα intestine-specific knockout (IKO) mice. Mouse offsprings carrying floxed AMPKα1 and AMPKα2, as well as vilERT2-Cre (tamoxifen-sensitive, ERT2-fused Cre recombinase expressed under the control of the villin promoter, for deletion of AMPKα in intestine), along with their wildtype littermates (carrying floxed AMPKα1 and AMPKα2, but not vilERT2-Cre) were injected with tamoxifen (as described in Method section) for knockout of AMPKα. Protein levels of AMPKα in duodenum were then analysed by immunoblotting. b, Intestinal AMPK is required for metformin to induce glucose lowering. Mice at 5 weeks old with intestinal AMPKα1/2 double knockout (AMPKα1/2-IKO), along with its wildtype littermates, were treated with metformin in drinking water (+ Met, 1 g/l) for 7 days (tamoxifen was injected at 4 weeks old). At day 8, mice were starved for 6 h, followed by oral glucose tolerance test (OGTT, results are shown as mean ± s.e.m., n = 7 for each genotype/treatment, except for WT mice treated with drinking water without metformin, n = 6; and P value by two-way RM ANOVA, followed by Tukey, compared blood glucose levels between WT + Met group and AMPKα1/2-IKO + Met group at the same time point; see also inset for AUC values, P value by two-way ANOVA, followed by Tukey). c, l, Strategies to generate PEN2-floxed (c) or ATP6AP1-floxed (l) allele. d, f, Depletion of PEN2 in the intestine or liver in PEN2-floxed mice. PCR analysis results of mouse offsprings carrying floxed PEN2, as well as vilERT2-Cre (d) or albERT2-Cre (f, same as in d, except Cre under albumin promoter, for deletion of PEN2 in liver) allele are shown on the upper panels. Protein levels of PEN2 in duodenal (d), or hepatic (f) tissues in PEN2 intestine- or liver-specific knockout (IKO or LKO) mice are also shown. See also panels of d and f for the experimental timeline of the analysis of phenotypes of PEN2-IKO (d) and PEN2-LKO (f) mice. e, Intestinal PEN2 is required for metformin-induced glucose-lowering effect. Mice were fed with HFD as in Fig. 4a. Serum insulin levels are shown as mean ± s.e.m., n = 5 for each genotype/treatment, and P value by two-way RM ANOVA, followed by Tukey, compared insulin levels between WT + Met group and PEN2-IKO + Met group at the same time point; see also inset for AUC values, P value by two-way ANOVA, followed by Tukey. g, h, PEN2 is required for metformin-induced reduction of hepatic fat. As depicted in f, mice at 38 weeks old with hepatic PEN2 knockout (PEN2-LKO), along with its wildtype littermates, were treated with metformin in drinking water (+ Met, 1 g/l) for 16 weeks. At week 54, mice were starved for 6 h, followed by intraperitoneal glucose tolerance test (g, IPGTT; serum insulin levels are shown as mean ± s.e.m., n = 5 for each genotype/treatment, and P values by two-way RM ANOVA, followed by Tukey, compared blood glucose or insulin levels between WT + Met group and PEN2-LKO + Met group at the same time point; see also inset for AUC values, P values by two-way ANOVA, followed by Tukey), insulin tolerance test (h, ITT, results are shown as mean ± s.e.m., n = 6 for each genotype/treatment, and P value by two-way RM ANOVA, followed by Tukey, compared as in c; see also inset for AUC values, P value by two-way ANOVA, followed by Tukey). i, p, The PEN2-ATP6AP1 axis is required for AMPK activation by metformin in primary hepatocytes from HFD-fed mice. PEN2-LKO mice were generated as in Fig. 4c (i), and ATP6AP1 or ATP6AP1Δ420-440 mutant was reintroduced to in the liver of mice lacking hepatic ATP6AP1 as in Fig. 4e (p), and the resultant mice were fed with HFD for 35 weeks. Primary hepatocytes were then isolated, and treated with 5 μM metformin or 500 μM metformin for 2 h, and then subjected to analysis p-AMPKα and p-ACC levels by immunoblotting. j, q, The PEN2-ATP6AP1 axis is required for AMPK activation in the liver of HFD-fed mice. Mice were treated as in Fig. 4c (j) or 4e (q). Hepatic AMPK activation (immunoblots), AMP:ATP and ADP:ATP ratios (scatter plots, left panel), as well as the absolute concentrations of AMP, ADP and ATP [scatter plots, middle panel, shown as mean ± s.e.m., n = 5 (j) and n = 5 (q) for each genotype/treatment, except for ischemic treatment, n = 4; P value by one-way ANOVA, followed by Tukey], and metformin concentrations [scatter plots, right panel, shown as mean ± s.e.m. on right panel, n = 6 (j) and n = 5 (q) for each genotype, and P value by two-sided Student’s t-test] in mice after 1-week treatment of metformin (1 g/l in drinking water) are shown. k, r, The PEN2-ATP6AP1 axis is required for the reduction of hepatic fat by metformin. Mice were fed with HFD as in Fig. 4c (k) or 4e (r), and images from H&E staining of the liver in mice after 16-week treatment of metformin are shown. m, Depletion of ATP6AP1, and re-introduction of ATP6AP1 or ATP6AP1Δ420-440 in liver in ATP6AP1-LKO mice. PCR analysis results of mouse offsprings carrying floxed ATP6AP1, as well as albERT2-Cre are shown on the upper panel. Protein levels of endogenous ATP6AP1, as well as full length (FL) ATP6AP1, or ATP6AP1Δ420-440 mutant expressed via AAVs, are shown on the middle panel. Experimental timeline of the analysis of the phenotypes of liver-specific ATP6AP1 (FL) and ATP6AP1Δ420-440-expressing mice were shown on the lower panel. n, o, ATP6AP1 is required for metformin-induced reduction of hepatic fat. As depicted in m, mice at 38 weeks old with hepatic depletion of ATP6AP1, and re-introduction of ATP6AP1 or ATP6AP1Δ420-440 (FL or Δ420-440) were treated with metformin in drinking water (+ Met, 1 g/l) for 16 weeks. At week 54, mice were starved for 6 h, followed by intraperitoneal glucose tolerance test (n, serum insulin levels are shown as mean ± s.e.m., n = 5 for each genotype/treatment, and P value by two-way RM ANOVA, followed by Tukey, compared insulin levels between ATP6AP1 (FL) + Met group and ATP6AP1Δ420-440 + Met group at the same time point; see also inset for AUC values, P value by two-way ANOVA, followed by Tukey), insulin tolerance test (o, shown as mean ± s.e.m., n = 6 for each genotype/treatment, and P value by two-way RM ANOVA, followed by Tukey, compared as in n; see also inset for AUC values, and P value by two-way ANOVA, followed by Tukey). Experiments in this figure were performed three times, except i four times.