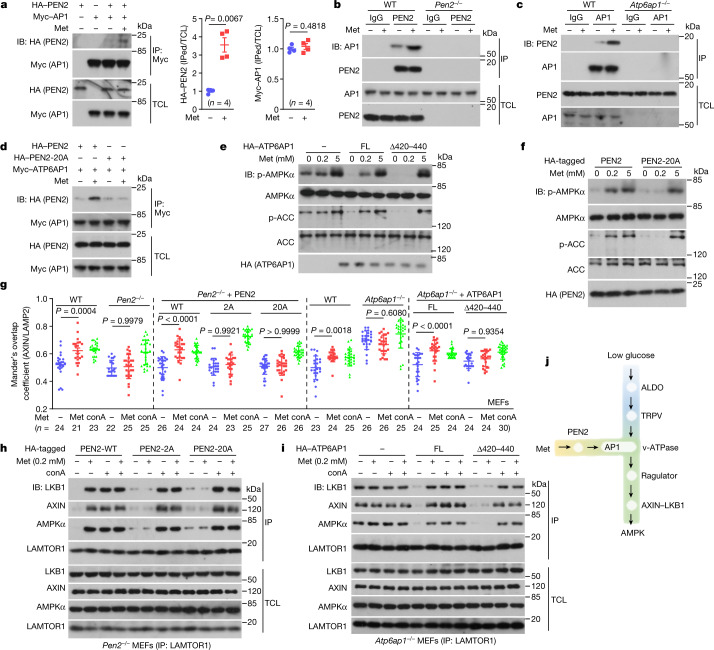
Fig. 3. ATP6AP1 tethers PEN2 to v-ATPase for AMPK activation.
a–c, Identification of ATP6AP1 as an interacting protein of PEN2. Lysates of HEK293T cells expressing HA–PEN2 or Myc–ATP6AP1 (a), and lysates from wild-type MEFs, Pen2–/– MEFs (b) or Atp6ap1–/– MEFs (c) were incubated with 10 μM metformin and immunoprecipitated (IP) for the PEN2 and AP1 proteins. d, Metformin does not promote the interaction between ATP6AP1 and PEN2-20A. HEK293T cells transfected with HA-tagged PEN2 or PEN2-20A were lysed and treated as in a. The interaction between ATP6AP1 and PEN2 was analysed by IP followed by IB. e–i, Loss of the PEN2–ATP6AP1 interaction abolishes the effects of metformin on AMPK activation. Atp6ap1–/– MEFs re-introduced with ATP6AP1Δ420–440 (e) or Pen2–/– MEFs re-introduced with the PEN2-20A mutant (f) were treated with 200 μM metformin for 12 h followed by analysis of p-AMPK and p-ACC. g–i, The effects of ATP6AP1 and PEN2 mutants on the lysosomal translocation of AXIN (g), and the formation of the AXIN-based complex (h, i) were analysed. Concanamycin A (conA; 5 μM for 2 h) was used as a control. FL, full length. j, A schematic depicting that the metformin–PEN2–ATP6AP1 and the FBP–aldolase axes constitute two incoming shunts that converge at v-ATPase to elicit AMPK activation through the lysosomal pathway. For gel source data, see Supplementary Fig. 1. Data are the mean ± s.e.m., n values are labelled on each panel, and P values were calculated using two-sided Student’s t-test (a, for Myc–ATP6AP1), two-sided Student’s t-test with Welch’s correction (a, for HA–PEN2) or two-way ANOVA, followed by Tukey’s test (g). Experiments in this figure were performed three times, except for a (four times), and h and i (five times).