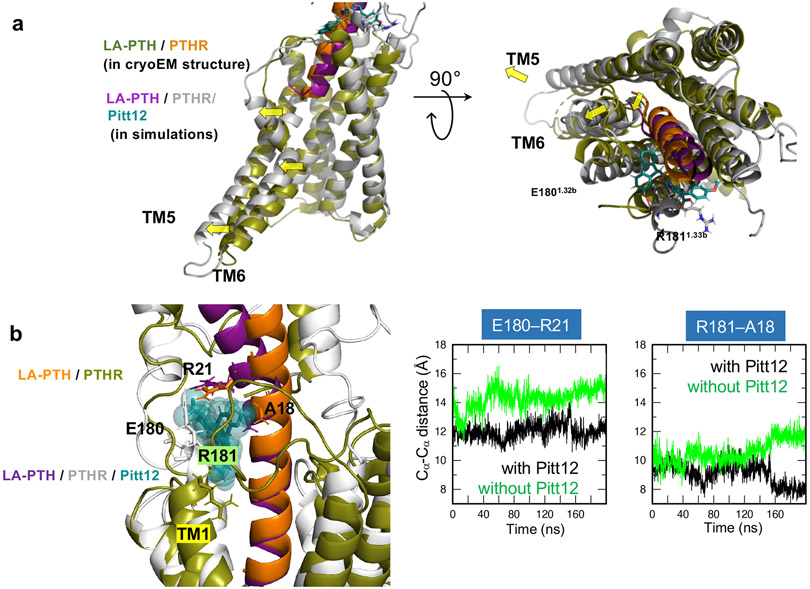
Figure 5. Conformational change of PTHR due to Pitt12 binding.
(a) Predicted Pitt12 binding-induced conformational changes in PTHR. N-terminal tip of LA-PTH pushes extracellular tip of TM6 further outwards leading to outward displacement of TM5 and TM6 helices.
(b) Comparison of the conformations stabilized in the MD runs performed for LA-PTH/PTHR in the presence and absence of Pitt12. Left, the superimposed final (t = 200 ns) snapshots from the two runs. Essential residues coordinating Pitt12 are shown in stick representation and labeled. Right, the time evolution of Cα-Cα distances between E180 of PTHR and R21 of LA-PTH (left panel) and between R181 of PTHR and A18 of LA-PTH (right panel) are presented, for the runs conducted in the presence (black) or in the absence of Pitt12 (green). The space between PTHR and LA-PTH is wider in the absence of Pitt12.