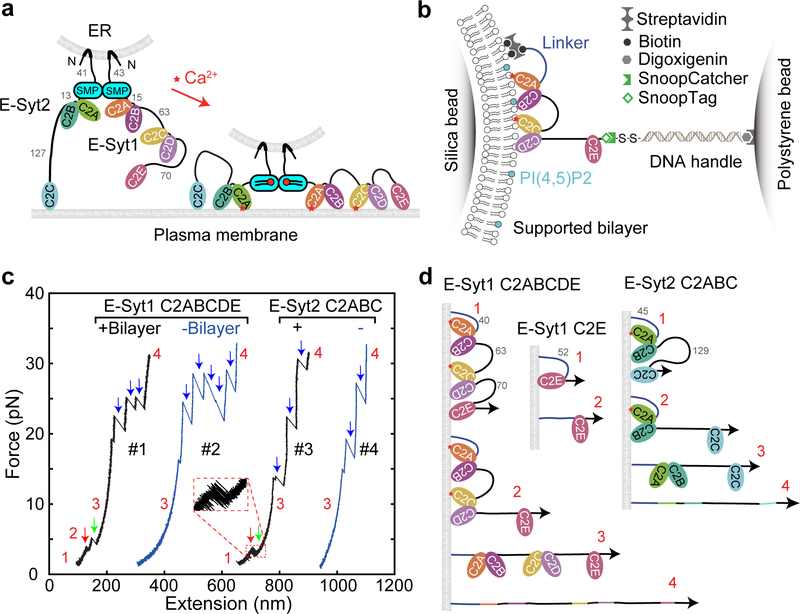
Fig. 1. E-Syt C2 domains bind membranes in a stepwise manner as revealed by optical tweezers.
(a) ER-anchored E-Syts form a dimer via their SMP domains and bind to the plasma membrane (PM) via their tandem C2 domains, pulling the ER-PM membrane close to facilitate lipid transfer in a Ca2+-dependent manner. The lengths of disordered linkers joining different C2 domains are indicated by their numbers in amino acids. (b) Schematics of the experimental setup to pull a single E-Syt1 C2 repeat C2ABCDE. (c) Force-extension curves (FECs) obtained by pulling single C2 repeats in the presence or absence of the lipid bilayer. Red and green arrows indicate stepwise C2 unbinding from the membrane, and black arrows denotes unfolding of individual C2 domains. (c) Schematics of different C2 binding states for some C2 domains or repeats tested in this study.