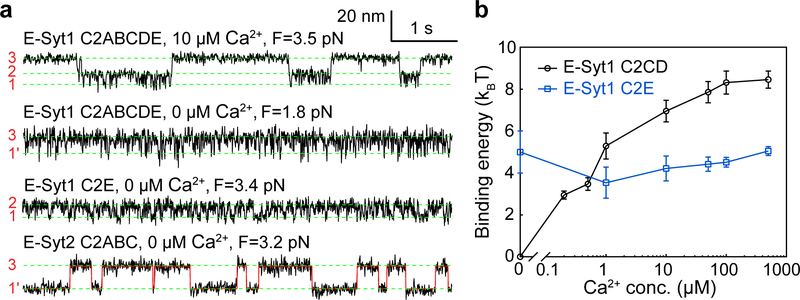
Fig. 4. Membrane binding of E-Syt1 C2CD and E-Syt2 C2AB is Ca2+-dependent, while binding of E-Syt1 C2E and E-Syt2 C2C is Ca2+-independent.
(a) Extension-time trajectories at constant force in different Ca2+ concentrations. (b) Unbinding free energy of E-Syt1 C2CD and C2E as a function of [Ca2+]. Each average energy value was determined by measurements from at least three different single molecules (n = 10 for 500 μM, 100 μM, 50 μM, 10 μM, n = 9 for 1 μM, n = 5 for 0.5 μM, n = 3 for 0.2 μM and 0 Ca2+, respectively). The n number varied as a single molecule broke during buffer changed in all probability. Data are presented as mean values +/− SEM.