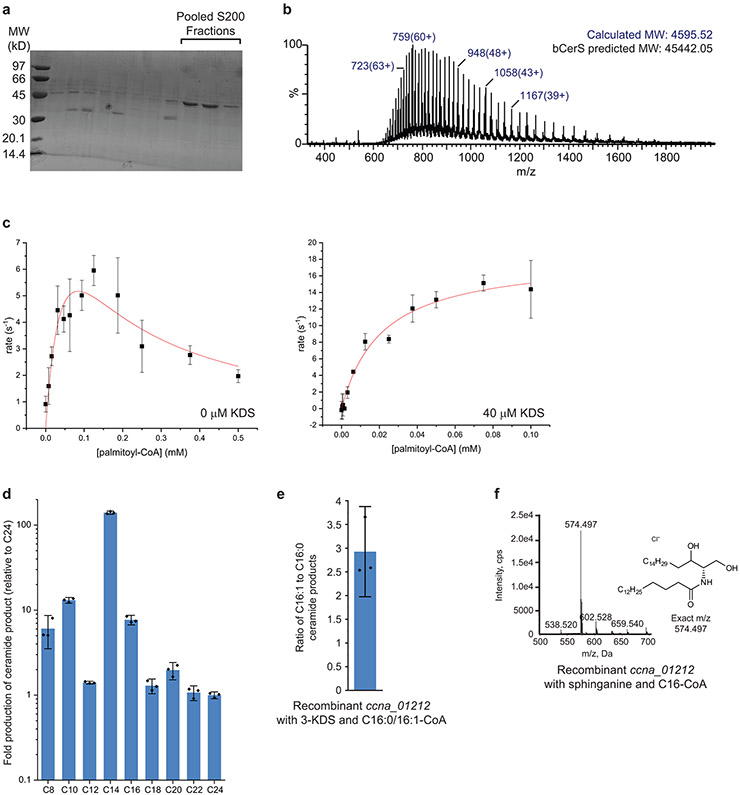
Extended Data Fig. 5. Purification of C. crescentus bCerS.
(a) bCerS was purified as described in the Materials and Methods. SDS-PAGE analysis confirmed protein purity following S200 size-exclusion chromatography. The indicated fractions were pooled for MS characterization. Recombinant protein was independently purified at least three times with similar purities as judged by Coomassie staining. (b) The identity of the purified bCerS protein was confirmed by LC/ESI/MS. The ions for two species were detected, which differed by 178 Da (top); this difference is due to spontaneous alpha-N-6-phosphogluconoylation which is commonly observed in His-tagged proteins expressed in E. coli41. The protein molecular weight was calculated using the relative abundances of the multiple-charged species of the protein analyte (bottom); the charges are indicated in parenthesis. (c) Kinetic analyses of CCNA_01212 in the absence (left) and presence (right) of 3-KDS determined the Km,app value for C16:0-CoA (21.3 ± 4.1 μM) (n=3, data are presented as mean +/− SD). (d) To determine the substrate specificity of C. crescentus bCerS, we incubated the recombinant enzyme with 40 μM 3-KDS and an equimolar mixture of fatty acid-CoA (C8-C24) with a total final concentration of 50 μM. The reaction proceeded for 1 hr and the reaction products were analyzed by ESI/MS. The integrated ion counts were normalized to C24 (n=3, data are presented as mean +/− SD). (e) To determine the acyl-chain saturation preference of C. crescentus bCerS, we incubated the recombinant enzyme with 40 μM 3-KDS and an equimolar mixture of C16:0-CoA and C16:1-CoA, with a total final concentration of 50 μM. The reaction proceeded for 1 hr and the reaction products were analyzed by NPLC-ESI/MS in the negative ion mode to determine the ratio of ceramide products produced (C16:0 product, [M+Cl]− at m/z 572.481; C16:1 product, [M+Cl]− at m/z 570.466) (n=3, data are presented as mean +/− SD). (f) Recombinant bCerS was incubated with 40 μM sphinganine and 50 μM C16:0-CoA for 1 hr and the reaction product was analyzed by ESI/MS.