Fig. 5.
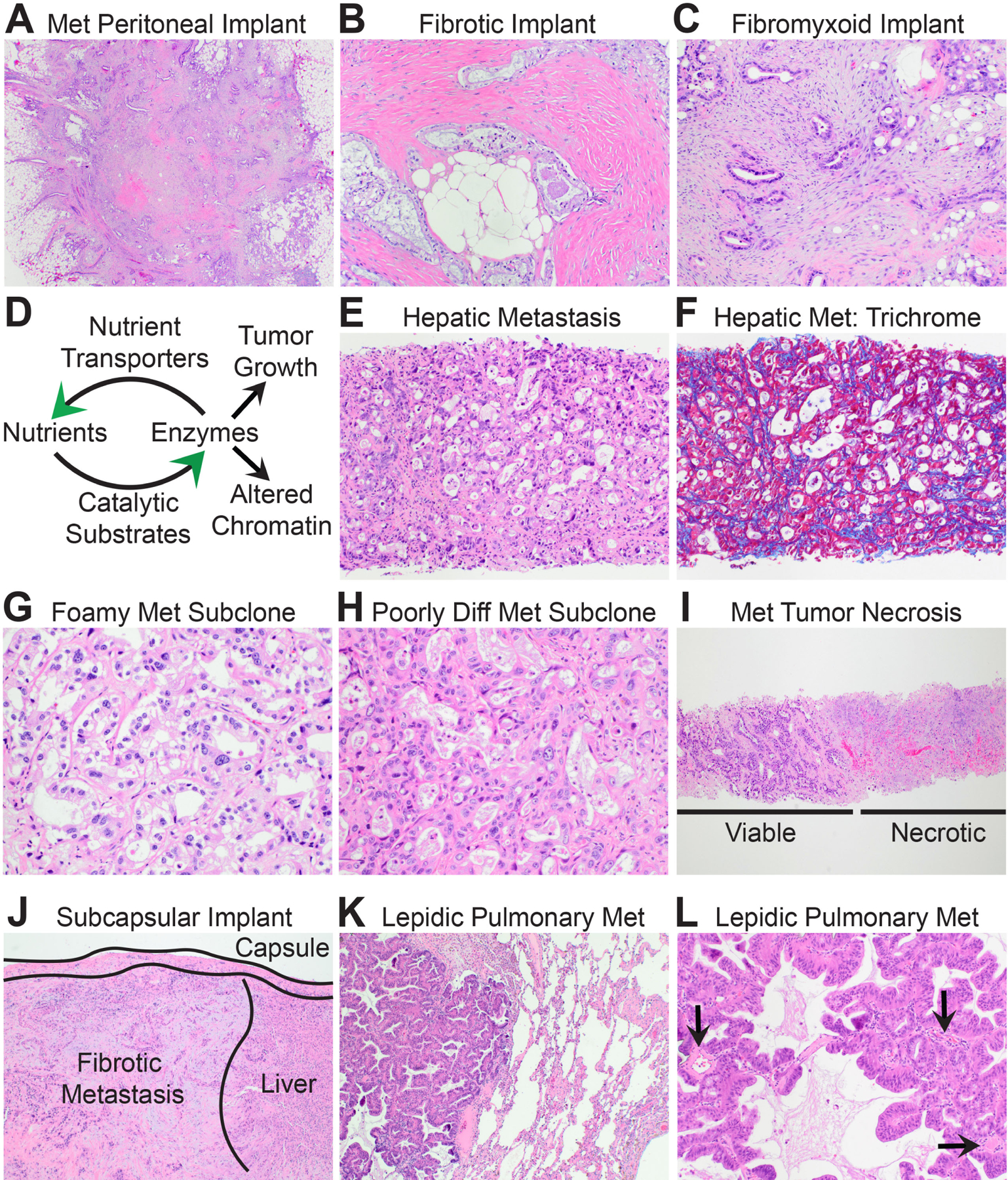
The biomorphology of metastatic PDAC. (A) Low power magnification shows that metastatic peritoneal implants resemble stellate scars. (B) Some peritoneal PDACs are encased within densely fibrotic stroma. (C) Others are encased within fibrofibromyxoid stroma. (D) Schematic illustrating positive feedback loops between nutrient transport systems and pro-tumourigenic biosynthetic enzymes that produce anabolic metabolites and/or reprogram chromatin for metastasis. (E) High power magnification of a distant metastatic PDAC with ‘biosynthetic’ morphology and an underdeveloped ‘delicate’ stroma. (F) Masson trichrome stain highlights the delicate pericellular fibrosis. (G) A liver metastasis seeded by a well-differentiated clear/foamy gland subclone forms back-to-back glands with delicate intervening stroma. (H) Likewise, a liver metastasis seeded by a poorly differentiated subclone is highly cellular with a delicate stroma. (I) In many instances, half of the core biopsy of a liver metastasis is viable tumour while the other half is necrotic. (J) Liver metastases may show well-developed fibrosis beneath the liver capsule. Such metastases are usually implants on the liver surface rather than true haematogenous metastases. (K) Low power magnification of a metastatic PDAC to the lung shows ‘lepidic’ growth within alveoli. (L) Like liver metastases, lepidic metastases develop a delicate stroma and glands are adjacent to native vessels (arrows).
