Abstract
BACKGROUND:
In the United States, approximately 52,000 women per year (accounting for 1.46% of births) experience severe maternal morbidity, which is defined as a complication that causes significant maternal harm or risk of death. It disproportionately affects women from racial or ethnic minorities, people with chronic diseases, and those with Medicaid or no insurance. Preconception care has been hailed as a strategy to improve pregnancy outcomes and reduce disparities, but its broad benefits for maternal outcomes have not been demonstrated.
OBJECTIVE:
Our objective was to measure the association between preconception care and the odds of severe maternal morbidity among women with Medicaid.
STUDY DESIGN:
This is a secondary analysis of Medicaid claims using the Medicaid Analytic Extract files (2010–2012). We used the International Classification of Diseases, Ninth Revision codes, published by the US Office of Population Affairs’ Quality Family Planning program to define 7 domains of preconception care. The primary outcome was maternal death within 12 weeks of delivery or severe maternal morbidity during birth hospitalization, defined by the presence of any diagnosis or procedure on the severe maternal morbidity International Classification of Diseases, Ninth Revision code list from the Centers for Disease Control and Prevention. Because this list may overestimate severe maternal morbidity by counting any blood transfusion, our secondary outcome used the same code list but without transfusion. We reviewed care in the year before conception and used logistic regression to estimate the association between each domain and severe maternal morbidity for all births to women enrolled in Medicaid and aged 15 to 45 years with births during 2012. We performed a subgroup analysis for women with chronic disease (kidney disease, hypertension, or diabetes).
RESULTS:
Severe maternal morbidity or death occurred in 26,285 births (1.74%) when including blood transfusions and 9,481 births (0.63%) when excluding transfusions. Receiving contraceptive services in the year before conception was associated with decreased odds of severe maternal morbidity (adjusted odds ratio, 0.92; 95% confidence interval, 0.88–0.95) and pregnancy test services were associated with increased odds (adjusted odds ratio, 1.08; 95% confidence interval, 1.01–1.14). In the primary analysis, no significant associations were observed for other preconception care domains. Among those women with at least 1 chronic disease, contraceptive care (adjusted odds ratio, 0.84; 95% confidence interval, 0.75–0.95) and routine physical or gynecologic exams (adjusted odds ratio, 0.79; 95% confidence interval, 0.71–0.88) were associated with decreased odds of severe maternal morbidity. Similar associations were found for severe maternal morbidity when excluding blood transfusion.
CONCLUSIONS:
Contraceptive services in the year before conception and routine exams for women with chronic disease are associated with decreased odds of severe maternal morbidity or death for Medicaid enrollees.
Keywords: contraception, maternal morbidity, Medicaid, preconception care
Introduction
In the United States, severe maternal morbidity (SMM), defined as a pregnancy complication that causes significant adverse consequences for the woman or puts her at risk of death,1 has increased nearly 200% in the past 2 decades, reaching a rate of 146 per 100,000 births in 2015.2 The rates of SMM in the United States are among the highest within developed countries.3 It disproportionately affects women from racial and ethnic minorities, residents of low-income zip codes, and people with chronic medical conditions.4–7
Previous research has shown that individual-level factors account for 20% to 40% of the variation in obstetrical complications.6 Hospital factors at the time of delivery account for only another 20%,8 suggesting that upstream factors contribute significantly to SMM. One such potential factor is preconception care, defined as preventative healthcare that a patient receives before pregnancy to address pregnancy-related risk factors. Preconception care is hailed as a promising strategy to prevent adverse pregnancy outcomes and reduce racial and ethnic disparities in infant health,9–11 but its benefits for maternal outcomes have not yet been demonstrated.
The Medicaid program covers almost half of all births in the United States, which is more than any other single payer.12,13 Women with Medicaid are more likely to experience SMM than women with private insurance.14 We used Medicaid claims data to examine the association between preconception care and the risk of SMM in this high-risk population, hypothesizing that women who utilize preconception care will be less likely to experience SMM in a subsequent pregnancy.
Materials and Methods
This is an analysis using the Medicaid Analytic Extract (MAX) data files from the Centers for Medicare and Medicaid Services (CMS) from 2010 to 2012, which we received under an approved Data Use Agreement. These data files include person-level information on Medicaid enrollees and encounter-level information for Medicaid claims from all sources of care, including inpatient, outpatient, physician services, radiology, clinic visits, and pharmacies. The University of Chicago’s Institutional Review Board approved this study.
We included all female beneficiaries aged 15 to 45 years who were enrolled in Medicaid for all states with data publicly available through CMS including Washington, DC (data were not available for the following states: AL, ID, ME, KS, RI, and SD) who experienced a delivery in 2012 and had Medicaid coverage for delivery of this index pregnancy. We identified deliveries using the following International Classification of Diseases, Ninth Revision (ICD-9) diagnosis codes: V27.xx with or without 650 for normal deliveries; and V27. xx with 644.2, 644.4, 765.0 or 765.1 for preterm births. For women with more than 1 delivery in calendar year 2012, we only used information from the first delivery.
The primary outcome was a composite of maternal death within 12 weeks of delivery or any SMM event during the delivery hospitalization (hereafter, “SMM” will refer to both severe maternal morbidity and maternal mortality). We chose to examine up to 12 weeks following delivery because of recent emphasis on the “fourth trimester,” the roughly 3 month period following pregnancy in which many complications arise.15 Further, up to 12% of maternal deaths occur after the 42-day period.16 We wanted to strike a balance between capturing some of these deaths in the later period and the fact that, practically, many women lose Medicaid coverage after 6 to 12 weeks postpartum, and thus, we would no longer be able to track them.
We identified women who experienced SMM using the ICD-9 diagnosis codes and Current Procedural Terminology codes that the Centers for Disease Control and Prevention (CDC) has compiled.17 This is a validated measure used in other studies on SMM.2,18 As a secondary outcome, we examined all women who experienced SMM apart from blood transfusion, as blood transfusion as the sole indicator of SMM often overestimates the true prevalence of SMM.19
The main exposure of interest was preconception care, which we defined as the receipt of specific healthcare services in the 12 months before conception of the 2012 index pregnancy. We first determined the date of conception using a modified version of the approach described by Palmsten et al.20 In brief, we calculated the date of conception to be 255 days before the delivery date of a full-term birth and 230 days before a premature birth.
We identified preconception care in the MAX outpatient files using a list of ICD 9 and 10 codes published by the United States Department of Health and Human Services (DHHS) under its Office of Population Affairs Quality Family Planning program.21 We utilized the following 7 domains of care, as previously defined by the CDC and DHHS: contraceptive services, pregnancy test & counseling, achieving pregnancy, basic infertility services, preconception health services, sexually transmitted infection services, and related preventative health services (which we refer to in this article as “routine physical or gynecologic exams,” as we feel that it describes more specifically what this domain encapsulates).22 In addition to each of the 7 domains listed above, we created a single binary variable indicating whether a woman had received at least ≥1 of these 7 types of preconception care (entitled “any preconception care”). Our group has previously utilized similar methods to examine SMM following ectopic pregnancy.23 The ICD-9 codes associated with each domain are in Supplemental Table 1. Of note, we excluded pregnancy tests and counseling billed within 30 days of the estimated date of conception of the index pregnancy.
We examined several variables as potential confounders. We used the MAX personal summary file to obtain the age at delivery and the maternal race or ethnicity (self-reported at the time of Medicaid enrollment). We determined whether women had a preterm delivery using the ICD-9 code from the delivery hospitalization (as above). Using information on the 12 months before conception for the index delivery, we determined whether women had a delivery within this period using the ICD-9 codes for the deliveries listed above. We constructed a binary covariate indicating interpregnancy interval < 12 months, and determined whether women experienced SMM in this previous delivery using the same criteria we used to identify SMM in the index delivery. We used data from the MAX chronic condition file to determine whether women had a chronic medical condition in either 2011 or 2012 (any cardiac comorbidity, diabetes, hypertension, chronic kidney disease, depression, chronic obstructive pulmonary disorder, or tobacco use). We used information from the index delivery to determine the mode of delivery (any vaginal delivery vs any cesarean delivery) and whether the pregnancy resulted in multiple births.
We used logistic regression to examine whether any preconception care was predictive of SMM, and then (in separate models) whether each of the 7 domains of preconception care were predictive of SMM. We adjusted for confounders in multivariable models. We included variables as confounders in the logistic regression on the basis of known or suspected risk factors for SMM based on previous literature, such as those described above.1,4,6 We calculated the adjusted odds ratios [aOR] and 95% confidence intervals [CI]. We used 2-sided hypothesis testing and considered the results significant at the P<.05 level. We fit all models using Stata Release 17 (StataCorp LLC, College Station, TX).
The only variables with missing data were race or ethnicity (“Unknown” in 67,169 cases or 4.4%, missing for 11 cases) and rural (missing for 1,508 cases, or 0.1%); in the first case “Unknown” was included as a separate category, whereas in the second case, these observations were omitted from the models including rural as a covariate.
We performed a subgroup analysis of women with at least 1 of the following 3 chronic diseases that place women at the highest risk of SMM: hypertension, diabetes, or chronic kidney disease.24 We also conducted a sensitivity analysis using a sample of women continuously eligible for Medicaid for the full 12 months before conception, as it is possible that women who were not eligible for Medicaid before conception may have received services that did not generate Medicaid billing claims and were thus not captured in our data.
Results
The Figure shows the total number of women aged 15 to 45 years enrolled in Medicaid in states with available data from 2012 and the number excluded for various reasons. Among 1,514,759 women with eligible deliveries in the final study sample, 26,285 women (1.74%) experienced SMM (9,481 women, 0.63%, when excluding blood transfusion). There were 198 deaths within 12 weeks of delivery. Women from racial and ethnic minorities were significantly more likely to experience the primary outcome, as were women who were older, had a preterm delivery, a short interval pregnancy, those who experienced SMM in a previous pregnancy, and those who had a medical comorbidity apart from tobacco use (Table 1).
TABLE 1.
Sample characteristics and incidence of severe maternal morbidity
| Characteristics | N | % | SMM (%)a | P-valueb |
|---|---|---|---|---|
| Total | 1,514,759 | 100.0 | 1.74 | |
| Age (y) | 1,514,759 | 25.7 (5.8)c | <.001d | |
| Race/ethnicity | <.001 | |||
| White non-Hispanic | 588,203 | 38.8 | 1.42 | |
| Black non-Hispanic | 321,824 | 21.3 | 2.56 | |
| Hispanic | 453,021 | 29.9 | 1.56 | |
| Asian, Pacific Islander or Native Hawaiian | 54,125 | 3.6 | 1.54 | |
| American Indian or Alaskan Native | 23,631 | 1.6 | 2.26 | |
| More than 1 race | 6,775 | 0.5 | 1.86 | |
| Unknown | 67,169 | 4.4 | 1.67 | |
| Rural residence | .289 | |||
| Yes | 281,221 | 18.6 | 1.71 | |
| No | 1,232,030 | 81.4 | 1.74 | |
| Previous SMM | <.001 | |||
| Yes | 2,098 | 0.1 | 12.25 | |
| No | 1,512,661 | 99.9 | 1.72 | |
| Interpregnancy interval <12 mo | <.001 | |||
| Yes | 124,828 | 8.2 | 1.92 | |
| No | 1,389,931 | 91.8 | 1.72 | |
| Preterm delivery | <.001 | |||
| Yes | 107,980 | 7.1 | 3.51 | |
| No | 1,406,779 | 92.9 | 1.60 | |
| Multiple births | <.001 | |||
| Yes | 15,659 | 1.0 | 5.38 | |
| No | 1,499,100 | 99.0 | 1.70 | |
| Cesarean delivery | <.001 | |||
| Yes | 413,900 | 27.3 | 3.46 | |
| No | 1,100,859 | 72.7 | 1.09 | |
| Any cardiometabolic comorbiditye | <.001 | |||
| Yes | 63,440 | 4.2 | 5.11 | |
| No | 1,451,319 | 95.8 | 1.59 | |
| Diabetes | <.001 | |||
| Yes | 24,113 | 1.6 | 3.44 | |
| No | 1,490,646 | 98.4 | 1.71 | |
| Hypertension | <.001 | |||
| Yes | 29,166 | 1.9 | 6.25 | |
| No | 1,485,593 | 98.1 | 1.65 | |
| Chronic kidney disease | <.001 | |||
| Yes | 16,246 | 1.1 | 8.19 | |
| No | 1,498,513 | 98.9 | 1.67 | |
| Depression | <.001 | |||
| Yes | 115,580 | 7.6 | 2.17 | |
| No | 1,399,179 | 92.4 | 1.70 | |
| Chronic obstructive pulmonary disease | <.001 | |||
| Yes | 4,033 | 0.3 | 3.47 | |
| No | 1,510,726 | 99.7 | 1.73 | |
| Tobacco use | .654 | |||
| Yes | 151,299 | 10.0 | 1.75 | |
| No | 1,363,460 | 90.0 | 1.73 |
SMM, severe maternal morbidity or mortality.
Percent of births within each group affected by SMM;
P value from chi-squared test of independence, unless otherwise noted;
Mean (standard deviation);
P value for 2-sample t test;
Includes diabetes, hypertension, and chronic kidney disease
Table 2 shows the predictors of any preconception care, any contraceptive care, and any related preventative health services in the year before the index delivery. Black non-Hispanic women were more likely to receive any preconception care, as were women with medical comorbidities, women who experienced a short interval pregnancy, and women who experienced SMM in a previous pregnancy. The findings were similar when examining the specific domains of any contraceptive care and any routine physical or gynecologic exam.
TABLE 2.
Prevalence of any preconception care, contraceptive care, and related preventative health services during the year before conception, overall and separately by demographic and clinical subgroups
| Comorbidity | Any preconception care | Contraceptive carea | Related preventative health servicesb | |||
|---|---|---|---|---|---|---|
| % | P-valuec | % | P-valuec | % | P-valuec | |
| Total | 22.9 | 12.3 | 11.2 | |||
| Race/ethnicity | <.001 | <.001 | <.001 | |||
| White non-Hispanic | 24.1 | 12.7 | 12.1 | |||
| Black non-Hispanic | 32.5 | 17.4 | 15.2 | |||
| Hispanic | 15.7 | 9.0 | 7.4 | |||
| Asian, Pacific Islander, or Native Hawaiian | 17.3 | 6.0 | 11.5 | |||
| American Indian or Alaskan Native | 28.4 | 16.5 | 10.3 | |||
| More than 1 race | 32.3 | 18.0 | 15.5 | |||
| Unknown | 17.2 | 8.6 | 9.0 | |||
| Rural residence | <.001 | <.001 | <.001 | |||
| Yes | 26.8 | 15.9 | 12.0 | |||
| No | 22.0 | 11.4 | 11.0 | |||
| Previous SMM | <.001 | <.001 | <.001 | |||
| Yes | 48.9 | 21.2 | 20.1 | |||
| No | 22.9 | 12.2 | 11.2 | |||
| Interpregnancy interval <12 mo | <.001 | <.001 | <.001 | |||
| Yes | 46.6 | 21.0 | 19.7 | |||
| No | 20.8 | 11.5 | 10.4 | |||
| Preterm delivery | <.001 | <.001 | <.001 | |||
| Yes | 26.2 | 13.5 | 12.5 | |||
| No | 22.7 | 12.2 | 11.1 | |||
| Multiple births | <.001 | <.001 | <.001 | |||
| Yes | 25.7 | 13.2 | 12.8 | |||
| No | 22.9 | 12.2 | 11.2 | |||
| Cesarean delivery | <.001 | <.001 | .491 | |||
| Yes | 22.4 | 11.7 | 11.2 | |||
| No | 23.1 | 12.5 | 11.2 | |||
| Any cardiometabolic comorbidityd | <.001 | <.001 | <.001 | |||
| Yes | 30.0 | 13.8 | 16.6 | |||
| No | 22.6 | 12.2 | 10.9 | |||
| Diabetes | <.001 | .905 | <.001 | |||
| Yes | 28.9 | 12.2 | 16.7 | |||
| No | 22.8 | 12.3 | 11.1 | |||
| Hypertension | <.001 | <.001 | <.001 | |||
| Yes | 30.3 | 13.7 | 17.2 | |||
| No | 22.8 | 12.2 | 11.1 | |||
| Chronic kidney disease | <.001 | <.001 | <.001 | |||
| Yes | 31.6 | 15.7 | 16.4 | |||
| No | 22.8 | 12.2 | 11.1 | |||
| Depression | <.001 | <.001 | <.001 | |||
| Yes | 40.0 | 20.2 | 21.1 | |||
| No | 21.5 | 11.6 | 10.4 | |||
| Chronic obstructive pulmonary disease | <.001 | <.001 | <.001 | |||
| Yes | 36.5 | 17.7 | 19.7 | |||
| No | 22.9 | 12.2 | 11.2 | |||
| Tobacco use | <.001 | <.001 | <.001 | |||
| Yes | 32.2 | 15.9 | 15.7 | |||
| No | 21.9 | 11.9 | 10.7 | |||
SMM, severe maternal morbidity or mortality.
Subset of “Any preconception care”;
Physical exam, gynecologic exam, or Pap smear; a subset of “Any preconception care”;
P value from chi-squared test of independence, unless otherwise noted;
Includes diabetes, hypertension, and chronic kidney disease.
Although any preconception care was associated overall with greater odds of SMM (odds ratio [OR], 1.09; 95% CI, 1.06–1.12), after adjusting for covariates it was associated with a reduction in the odds of SMM (aOR, 0.97; 95% CI, 0.95–1.00) and in the odds of SMM excluding transfusions only (aOR, 0.93; 95% CI, 0.89–0.98) (Tables 3 and 4). This effect was greatest for contraceptive services, which was associated with a reduction in the odds of SMM of 8% (aOR, 0.92; 95% CI, 0.88–0.95) and, excluding transfusions only, of 17% (aOR, 0.83; 95% CI, 0.78–0.89). In contrast, pregnancy testing and counseling were associated with an increase in the odds of SMM (aOR, 1.08; 95% CI, 1.01–1.14) and in the odds of SMM excluding transfusions only (aOR, 1.14; 95% CI, 1.04–1.26). Previous SMM remained highly associated with SMM in the current pregnancy, as did preterm birth and having multiple births. Women from racial and ethnic minorities were also more likely to experience SMM than White women. Although cesarean delivery was associated with higher odds of SMM, including it as a covariate did not alter the odds ratios for any preconception care or its subdomains (not shown).
TABLE 3.
Logistic regressions of severe maternal morbidity on measures of preconception care
| Variables | Model (1) | Model (2) | Model (3) | Model (4) | ||||
|---|---|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | Odds ratio | 95% CI | Odds ratio | 95% CI | Odds ratio | 95% CI | |
| Covariate | ||||||||
| Any preconception care | 1.09 | (1.06–1.12) | 0.97 | (0.95–1.00) | ||||
| Contraceptive services | 0.95 | (0.91–0.98) | 0.92 | (0.8–80.95) | ||||
| Pregnancy testing and counseling | 1.15 | (1.08–1.21) | 1.08 | (1.01–1.14) | ||||
| Achieving pregnancy | 1.37 | (0.82–2.29) | 1.29 | (0.76–2.19) | ||||
| Infertility services | 1.07 | (0.83–1.37) | 0.95 | (0.74–1.22) | ||||
| Preconception health services | 1.05 | (0.93–1.20) | 0.96 | (0.84–1.10) | ||||
| Sexually transmitted infection services | 1.11 | (1.05–1.17) | 1.00 | (0.95–1.05) | ||||
| Routine physical or gynecologic exams | 1.05 | (1.01–1.09) | 0.99 | (0.95–1.03) | ||||
| Maternal age (decades) | 0.98 | (0.95–1.00) | 0.97 | (0.95–1.00) | ||||
| Maternal age squared | 1.25 | (1.22–1.28) | 1.25 | (1.22–1.28) | ||||
| Short interpregnancy interval (<12 mo) | 0.98 | (0.94–1.03) | 0.98 | (0.94–1.03) | ||||
| Previous SMM | 6.78 | (5.89–7.81) | 6.77 | (5.88–7.80) | ||||
| Preterm birth | 1.79 | (1.72–1.86) | 1.79 | (1.72–1.85) | ||||
| Race/ethnicity | ||||||||
| White non-Hispanic | 1.00 | 1.00 | ||||||
| Black non-Hispanic | 1.72 | (1.66–1.78) | 1.72 | (1.67–1.78) | ||||
| Hispanic | 1.14 | (1.10–1.18) | 1.14 | (1.11–1.18) | ||||
| Asian, Pacific Islander, or Native Hawaiian | 1.13 | (1.05–1.22) | 1.13 | (1.05–1.21) | ||||
| American Indian or Alaskan Native | 1.60 | (1.47–1.75) | 1.60 | (1.46–1.75) | ||||
| More than 1 race | 1.29 | (1.08–1.54) | 1.29 | (1.08–1.54) | ||||
| Unknown | 1.18 | (1.11–1.26) | 1.18 | (1.11–1.26) | ||||
| Diabetes | 1.22 | (1.13–1.32) | 1.22 | (1.13–1.32) | ||||
| Hypertension | 2.64 | (2.51–2.79) | 2.64 | (2.51–2.79) | ||||
| Chronic kidney disease | 4.02 | (3.78–4.27) | 4.02 | (3.78–4.27) | ||||
| Depression | 1.18 | (1.13–1.24) | 1.18 | (1.13–1.23) | ||||
| Chronic obstructive pulmonary disease | 1.30 | (1.09–1.56) | 1.30 | (1.09–1.56) | ||||
| Tobacco use | 0.97 | (0.93–1.01) | 0.97 | (0.93–1.01) | ||||
| Rural residence | 1.05 | (1.02–1.09) | 1.06 | (1.02–1.09) | ||||
| Multiple births | 2.46 | (2.28–2.64) | 2.46 | (2.28–2.64) | ||||
CI, confidence interval; SMM, severe maternal morbidity.
TABLE 4.
Logistic regressions of severe maternal morbidity, excluding blood transfusions, on measures of preconception care
| Variable | Model (1) | Model (2) | Model (3) | Model (4) | ||||
|---|---|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | Odds ratio | 95% CI | Odds ratio | 95% CI | Odds ratio | 95% CI | |
| Covariate | ||||||||
| Any preconception care | 1.03 | (0.98–1.08) | 0.93 | (0.89–0.98) | ||||
| Contraceptive services | 0.82 | (0.77–0.88) | 0.83 | (0.78–0.89) | ||||
| Pregnancy testing and counseling | 1.20 | (1.09–1.32) | 1.14 | (1.04–1.26) | ||||
| Achieving pregnancy | 1.28 | (0.53–3.10) | 1.04 | (0.41–2.66) | ||||
| Infertility services | 1.11 | (0.74–1.66) | 0.89 | (0.59–1.33) | ||||
| Preconception health services | 1.14 | (0.93–1.40) | 1.04 | (0.84–1.28) | ||||
| Sexually transmitted infection services | 1.03 | (0.94–1.13) | 0.98 | (0.90–1.07) | ||||
| Routine physical/gynecologic exams | 1.11 | (1.04–1.19) | 0.99 | (0.93–1.06) | ||||
| Maternal age (decades) | 1.18 | (1.13–1.23) | 1.18 | (1.13–1.23) | ||||
| Maternal age squared | 1.17 | (1.12–1.23) | 1.17 | (1.12–1.22) | ||||
| Short interpregnancy interval (<12 mo) | 0.80 | (0.73–0.87) | 0.79 | (0.72–0.86) | ||||
| Previous SMM | 6.37 | (5.00–8.10) | 6.35 | (4.99–8.07) | ||||
| Preterm birth | 1.95 | (1.84–2.06) | 1.94 | (1.83–2.06) | ||||
| Race/ethnicity | ||||||||
| White non-Hispanic | 1.00 | 1.00 | ||||||
| Black non-Hispanic | 1.43 | (1.35–1.50) | 1.43 | (1.35–1.51) | ||||
| Hispanic | 0.99 | (0.94–1.05) | 0.99 | (0.94–1.05) | ||||
| Asian, Pacific Islander, or Native Hawaiian | 0.98 | (0.87–1.11) | 0.98 | (0.87–1.11) | ||||
| American Indian or Alaskan Native | 1.43 | (1.23–1.66) | 1.42 | (1.22–1.66) | ||||
| More than 1 race | 0.94 | (0.68–1.31) | 0.94 | (0.68–1.31) | ||||
| Unknown | 1.14 | (1.03–1.26) | 1.14 | (1.03–1.26) | ||||
| Diabetes | 1.13 | (1.01–1.27) | 1.13 | (1.01–1.26) | ||||
| Hypertension | 3.50 | (3.25–3.78) | 3.50 | (3.25–3.77) | ||||
| Chronic kidney disease | 7.63 | (7.09–8.22) | 7.63 | (7.08–8.21) | ||||
| Depression | 1.24 | (1.15–1.32) | 1.23 | (1.15–1.32) | ||||
| Chronic obstructive pulmonary disease | 1.48 | (1.17–1.89) | 1.48 | (1.16–1.89) | ||||
| Tobacco use | 0.99 | (0.93–1.06) | 0.99 | (0.92–1.06) | ||||
| Rural residence | 0.99 | (0.94–1.05) | 0.99 | (0.94–1.05) | ||||
| Multiple births | 1.18 | (1.01–1.38) | 1.18 | (1.01–1.38) | ||||
CI, confidence interval; SMM, severe maternal morbidity.
In our subgroup analysis of women with hypertension, diabetes, or chronic kidney disease (n=63,440), any preconception care was associated with a decrease in the odds of SMM (aOR, 0.84; 95% CI, 0.77–0.91), and contraceptive care remained associated with decreased odds of SMM (aOR, 0.84; 95% CI, 0.75–0.95). In addition, routine physical or gynecologic exams were also associated with a decrease in the odds of SMM (aOR, 0.79; 95% CI, 0.71–0.88). These results were nearly identical when excluding transfusions only.
Finally, a sensitivity analysis including only the 593,887 women continuously eligible for Medicaid during the year before conception showed findings similar to the overall cohort.
Discussion
Principal findings
Receiving any preconception care is associated with a modestly decreased risk of SMM when excluding blood transfusions and after adjusting for multiple potential confounders. Contraceptive services, which is 1 domain of preconception care in the year before an index delivery, is significantly associated with decreased odds of SMM. Routine physical or gynecologic exams among women with chronic disease were also associated with decreased odds of SMM. These findings provide concrete evidence of the value of preconception care. Conversely, after adjusting for other service domains and covariates, having an in-office pregnancy test was associated with increased odds of SMM.
Like Admon et al,18 we also found substantial disparities in the likelihood of SMM on the basis of maternal race and ethnicity, with women from minority groups being more likely to experience SMM. We found that although Black non-Hispanic women and women from some other racial minorities were actually more likely to receive preconception care, they, nonetheless, had a higher risk of SMM, suggesting that 1 or more factors we could not measure (including possibly systematic racism) may play an important role in outcomes. Hispanic women, conversely, were less likely to have received preconception care than White non-Hispanic women; this could lead to poorer outcomes, particularly as these women are also more likely to have a chronic health condition,25 which increases the risk of SMM.4 These findings are also in keeping with national data, which show substantially higher rates of SMM and maternal mortality among women from racial and ethnic minorities.26
Finally, women with chronic diseases, including diabetes, chronic hypertension, and chronic kidney disease, were substantially more likely to experience SMM, which confirms findings from previous studies.24,27
Clinical and research implications
To date, there has not been much concrete evidence of the value of preconception care; our study shows that certain aspects of preconception care may improve obstetrical outcomes. Contraceptive services are associated with a decreased risk of SMM in our study, perhaps, in part, because contraception facilitates women’s abilities to plan the timing of their pregnancies. Previous research has found unwanted and mistimed pregnancies associated with a delay in initiating prenatal care and other markers of poor perinatal health.28 This may be particularly important for women with chronic diseases such as diabetes, who can use contraception to gain time to get their chronic conditions under better control before getting pregnant.25 Physical or gynecologic exams for women with chronic disease may offer an opportunity to discuss optimizing care for that disease before conception. These findings lend credence to the idea that all providers who see women of reproductive age may play a role in discussing potential pregnancies with their patients, not just clinicians seeing patients specifically for the purposes of pregnancy planning or prevention.
An alternate explanation for these findings could be that women who use preconception care are in general more concerned with their health or are different from women who do not use preconception care (or have access to preconception care) in ways that affect SMM (such as poorer baseline health beyond the diagnoses we could identify). Thus, the preconception care may be a proxy for other factors associated with improved outcomes rather than the cause. Future research could build on this observational study by examining whether interventions aimed at increasing access to preconception care, including recent changes to the Medicaid eligibility criteria in several states, decrease the risk of SMM. We also found that women who access pregnancy tests and counseling are more likely to experience SMM; whether this is a marker of some other characteristic (such as being less likely to plan a pregnancy) warrants further investigation.
Access to care is based on more than just insurance eligibility; women who live in rural areas, for instance, may face physical barriers to accessing both preconception and high-quality obstetrical care in ways that affect SMM.29 Other analyses could focus on clustering of preconception care and SMM at the neighborhood, regional, or state level to determine whether there are barriers to preconception care beyond insurance coverage.
Strengths and limitations
This study has several strengths, including a large, nationwide, ethnically and racially diverse sample. Women in the Medicaid population are at a higher risk of pregnancy complications than women who are privately insured.30 Thus, this study focuses on a population at a high risk of SMM. Even within this population, SMM remains a rare event. Thus, our sample size is a strength.
This study has several limitations. We could not determine the content of preconception care, only that a woman had a visit. We could not know whether she was specifically counseled regarding pregnancy risks and preconception behaviors that may improve outcomes. Despite controlling for confounders, there may remain endogeneity in terms of which women seek out preconception care in ways that also affect the risk of SMM, and residual confounding may remain. We also could not observe the care that women may have received via avenues other than those funded through Medicaid, including during periods covered by private insurance, care paid for out-of-pocket, or care received through clinics free at the point of care. There may also be ascertainment bias because of the fact that all women were not covered continuously by Medicaid, though our sensitivity analyses did not show a significant difference in results when examining only women who were continuously covered through the preconception period. Although people of all gender identities can get pregnant, we specifically limited our sample to Medicaid recipients who identified as female, as Medicaid claims data do not have a consistent way to interrogate whether pregnancies among male recipients are errors or are pregnancies in individuals who identify as male. Although this sample is large and diverse in many ways, all patients in this study were insured by Medicaid. Thus, the findings may not apply to the larger obstetrical population with private or other government insurance or women without insurance. Further, not all states were included, as not all states had available data, potentially further limiting generalizability. As we included deliveries through the end of 2012 but the data were limited or largely unavailable through 2013, we have likely undercounted the postpartum maternal morbidity and mortality events for deliveries from October 2012 to December 2012. Finally, our odds ratios, though significant, showed a relatively small effect size.
FIGURE 1. Eligibility flowchart.
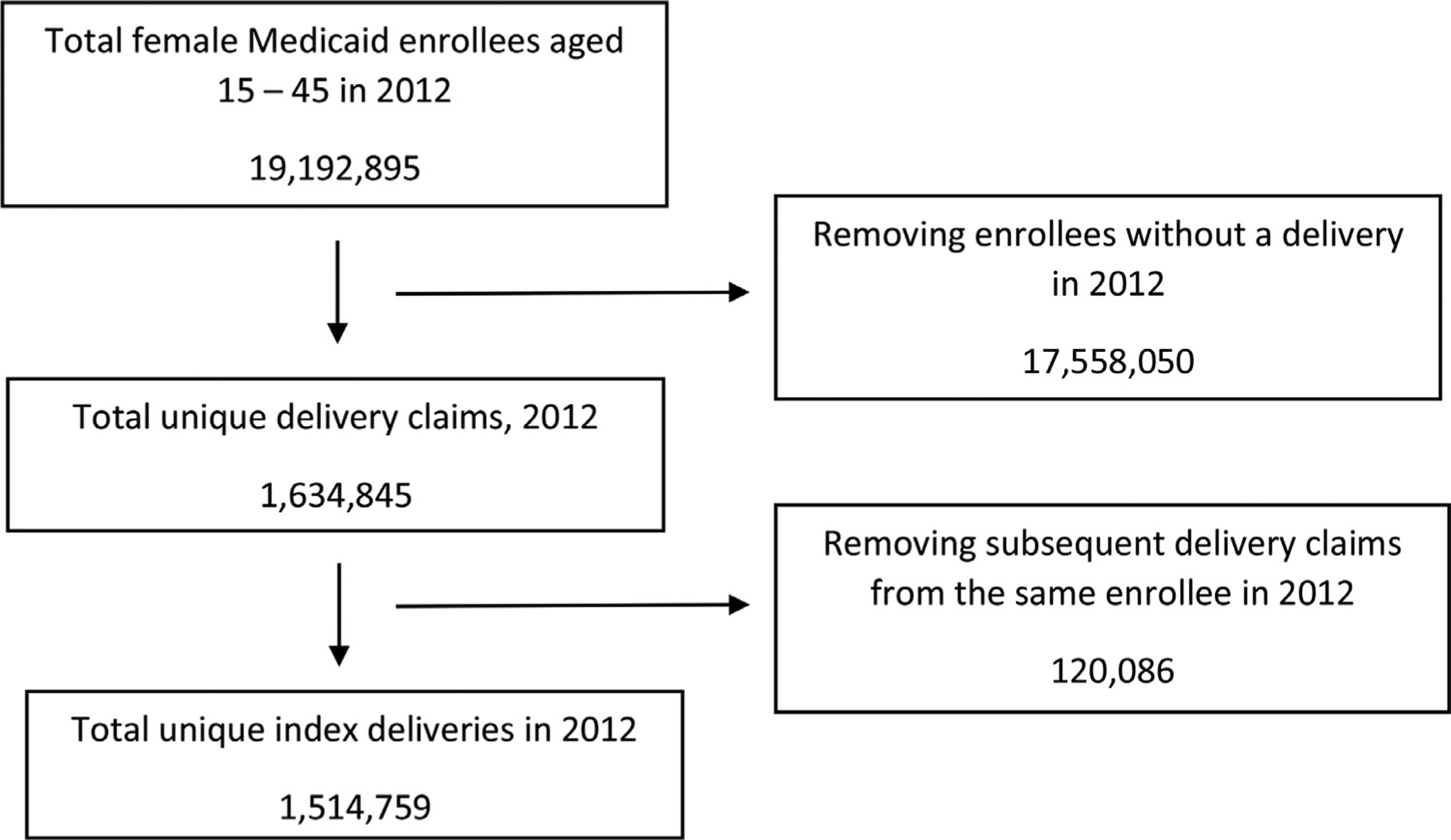
A flowchart showing the number of total female Medicaid beneficiaries aged 15 to 45 years in 2012, total unique deliveries in 2012, and total unique index deliveries in 2012.
Conclusion
Controlling for known risk factors; receiving preconception care, especially contraceptive services in the year before conception; and routine exams for women with chronic disease are associated with decreased odds of SMM for Medicaid enrollees.
Supplementary Material
AJOG MFM at a Glance.
Why was this study conducted?
To determine whether preconception care was associated with a reduction in the incidence of severe maternal morbidity or mortality during delivery hospitalization for a subsequent pregnancy.
Key findings
Contraceptive services in the year before conception and routine exams for women with chronic disease are associated with decreased odds of severe maternal morbidity or mortality at the time of delivery among Medicaid enrollees.
What does this add to what is known?
This study emphasizes the potential role of preconception care in improving obstetrical outcomes among pregnant women enrolled in Medicaid, which is a population at a high risk of severe maternal morbidity and mortality.
ACKNOWLEDGMENTS
The authors would like to acknowledge Anup Patel, BS, for contributions to earlier drafts of this work.
This work was supported by Agency for Healthcare Research and Quality (grant number R03 HS27027-01).
Footnotes
The authors report no conflict of interest.
Supplementary materials
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ajogmf.2021.100549.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This paper was presented at the annual meeting of the North American Primary Care Research Group, held virtually, November 20–24, 2020.
References
- 1.Callaghan WM, Creanga AA, Kuklina EV. Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstet Gynecol 2012;120:1029–36. [DOI] [PubMed] [Google Scholar]
- 2.Fingar KR, Hambrick MM, Heslin KC, Moore JE. Trends and disparities in delivery hospitalizations involving severe maternal morbidity, 2006–2015: statistical brief #243. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD): Agency for Healthcare Research and Quality (US); 2018. September 4. [PubMed] [Google Scholar]
- 3.World Health Organization. Trends in maternal mortality: 1990–2015: estimates from WHO, UNICEF, UNFPA, World Bank Group and the United Nations Population Division. World Health Organization; 2015. [Google Scholar]
- 4.Creanga AA, Bateman BT, Kuklina EV, Callaghan WM. Racial and ethnic disparities in severe maternal morbidity: a multistate analysis, 2008–2010. Am J Obstet Gynecol 2014;210: 435.. e1–8. [DOI] [PubMed] [Google Scholar]
- 5.Creanga AA, Berg CJ, Syverson C, Seed K, Bruce FC, Race Callaghan WM. ethnicity, and nativity differentials in pregnancy-related mortality in the United States: 1993–2006. Obstet Gynecol 2012;120:261–8. [DOI] [PubMed] [Google Scholar]
- 6.Grobman WA, Bailit JL, Rice MM, et al. Frequency of and factors associated with severe maternal morbidity. Obstet Gynecol 2014;123: 804–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grobman WA, Bailit JL, Rice MM, et al. Racial and ethnic disparities in maternal morbidity and obstetric care. Obstet Gynecol 2015;125:1460–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grobman WA, Bailit JL, Rice MM, et al. Can differences in obstetric outcomes be explained by differences in the care provided? The MFMU Network APEX study. Am J Obstet Gynecol 2014;211:147.e1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atrash HK, Johnson K, Adams M, Cordero JF, Howse J. Preconception care for improving perinatal outcomes: the time to act. Matern Child Health J 2006;10(Suppl 5):S3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson K, Posner SF, Biermann J, et al. Recommendations to improve preconception health and health care—United States. A report of the CDC/ATSDR Preconception Care Work Group and the Select Panel on Preconception Care. MMWR Recomm Rep 2006;55(RR-6):1–23. [PubMed] [Google Scholar]
- 11.Howell EA, Brown H, Brumley J, et al. Reduction of peripartum racial and ethnic disparities: a conceptual framework and maternal safety consensus bundle. Obstet Gynecol 2018;131:770–82. [DOI] [PubMed] [Google Scholar]
- 12.Kathleen Gifford, et al. View from the States: Key Medicaid Policy Changes. San Francisco, CA: Kaiser Family Foundation; 2019. [Google Scholar]
- 13.Markus AR, Andres E, West KD, Garro N, Pellegrini C. Medicaid covered births, 2008 through 2010, in the context of the implementation of health reform. Womens Health Issues 2013;23:e273–80. [DOI] [PubMed] [Google Scholar]
- 14.Howell EA, Egorova NN, Janevic T, et al. Race and ethnicity, medical insurance, and within-hospital severe maternal morbidity disparities. Obstet Gynecol 2020;135:285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamilton N, Stevens N, Lillis T, Adams N. The fourth trimester: toward improved postpartum health and healthcare of mothers and their families in the United States. J Behav Med 2018;41:571–6. [DOI] [PubMed] [Google Scholar]
- 16.Petersen EE, Davis NL, Goodman D, et al. Vital signs: pregnancy-related deaths, United States, 2011–2015, and strategies for prevention, 13 states, 2013–2017. MMWR Morb Mortal Wkly Rep 2019;68:423–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Severe maternal morbidity in the United States. 2020. Available at: https://www.cdc.gov/reproductivehealth/maternalinfanthealth/smm/severe-morbidity-ICD.htm. Accessed November 23, 2021.
- 18.Admon LK, Winkelman TNA, Zivin K, Terplan M, Mhyre JM, Dalton VK. Racial and ethnic disparities in the incidence of severe maternal morbidity in the United States, 2012–2015. Obstet Gynecol 2018;132:1158–66. [DOI] [PubMed] [Google Scholar]
- 19.Main EK, Abreo A, McNulty J, et al. Measuring severe maternal morbidity: validation of potential measures. Am J Obstet Gynecol 2016;214:643.. e1–10. [DOI] [PubMed] [Google Scholar]
- 20.Palmsten K, Huybrechts KF, Mogun H, et al. Harnessing the Medicaid analytic eXtract (MAX) to evaluate medications in pregnancy: design considerations. Plos One 2013;8: e67405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reproductive Health National Training Center. Crosswalk for frequently used quality family planning ICD codes. 2017. Available at: https://rhntc.org/resources/crosswalk-frequently-used-quality-family-planning-icd-codes. Accessed November 23, 2021.
- 22.Gavin L, Moskosky S, Carter M, et al. Providing quality family planning services: recommendations of CDC and the US Office of Population Affairs. MMWR Recomm Rep 2014;63(RR-04):1–54. [PubMed] [Google Scholar]
- 23.Stulberg DB, Cain L, Dahlquist IH, Lauderdale DS. Ectopic pregnancy morbidity and mortality in low-income women, 2004–2008. Hum Reprod 2016;31:666–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Admon LK, Winkelman TNA, Heisler M, Dalton VK. Obstetric outcomes and delivery-related health care utilization and costs among pregnant women with multiple chronic conditions. Prev Chronic Dis 2018;15:E21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fridman M, Korst LM, Chow J, Lawton E, Mitchell C, Gregory KD. Trends in maternal morbidity before and during pregnancy in California. Am J Public Health 2014;104(Suppl 1): S49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Louis JM, Menard MK, Gee RE. Racial and ethnic disparities in maternal morbidity and mortality. Obstet Gynecol 2015;125:690–4. [DOI] [PubMed] [Google Scholar]
- 27.Brown CC, Adams CE, George KE, Moore JE. Associations between comorbidities and severe maternal morbidity. Obstet Gynecol 2020;136:892–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng D, Schwarz EB, Douglas E, Horon I. Unintended pregnancy and associated maternal preconception, prenatal and postpartum behaviors. Contraception 2009; 79:194–8. [DOI] [PubMed] [Google Scholar]
- 29.Kozhimannil KB, Interrante JD, Henning-Smith C, Admon LK. Rural-urban differences in severe maternal morbidity and mortality in the US, 2007–15. Health Aff (Millwood) 2019;38: 2077–85. [DOI] [PubMed] [Google Scholar]
- 30.Wang E, Glazer KB, Howell EA, Janevic TM. Social determinants of pregnancy-related mortality and morbidity in the United States: a systematic review. Obstet Gynecol 2020;135: 896–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


