Figure 3. Perturbation of GLR signaling improves regeneration.
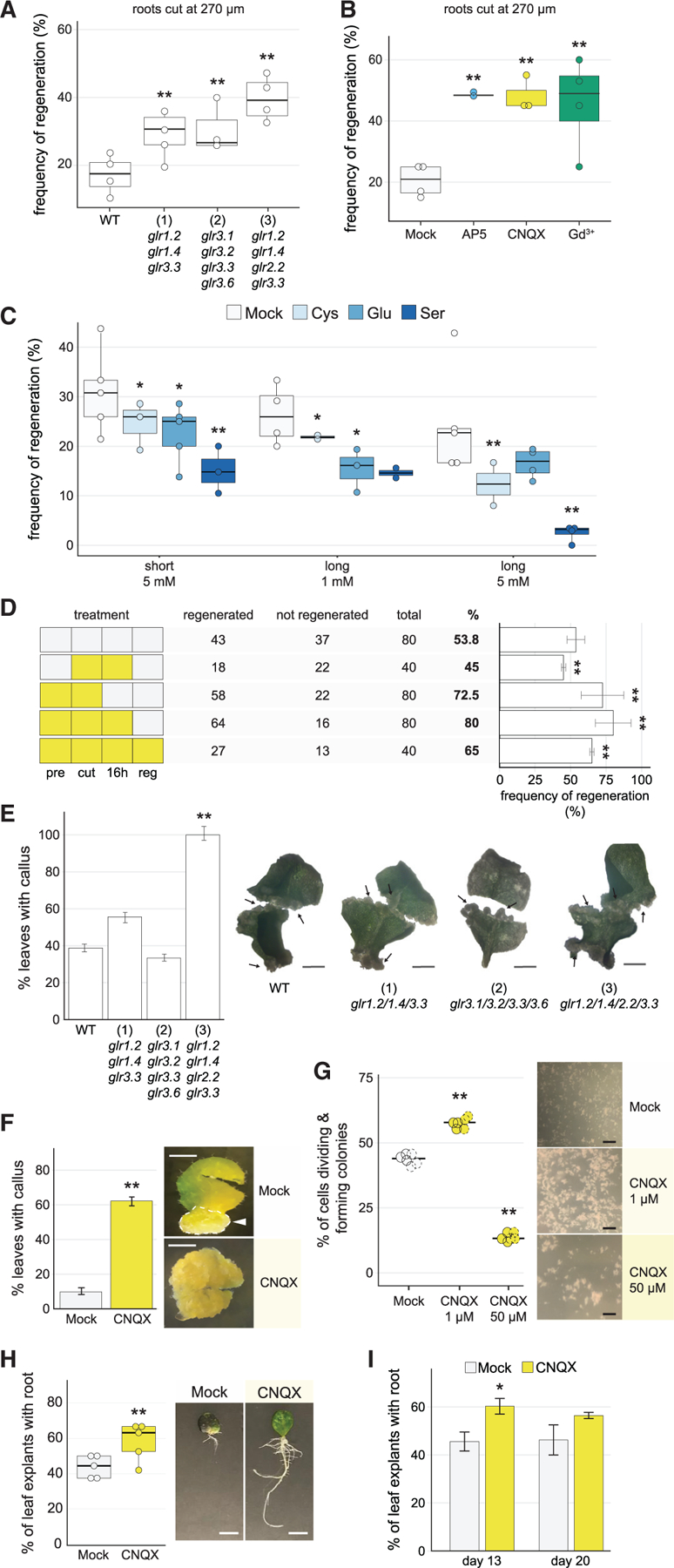
(A) Regeneration frequency of wild-type plants (Col-0) compared with triple and quadruple glr mutants: (1) glr1.2/1.4/3.3, (2) glr3.1/3.2/3.3/3.6, and (3) glr1.2/1.4/2.2/3.3 (chi-square test, Fisher’s combined probability test; n > 19 for each genotype in each of 4 trials; **p < 10–7).
(B) Constitutive post-injury treatment (cut on treatment plates and kept on treatment plates for the duration of the experiment) of cut roots on various calcium signaling antagonists, showing enhanced regeneration on all treatments. Roots were grown on normal media, cut, and transferred to treatment plates (chi-square test, Fisher’s combined probability test; n > 20 for each treatment in each of 4 trials, Gd3+ n > 12 one trial, **p < 10–11).
(C) Regeneration frequency in Col-0 plants treated with different amino acid agonists for GLRs. Regeneration frequencies were diminished in all treatments (chi-square test, Fisher’s combined probability test; n > 25 for each agonist treatment in each condition, 6 trials short treatment, 4 trials long treatment, *p < 0.02, **p < 10–6). Roots were grown on standard media, cut, and transferred to agonists. For ‘‘short’’ treatment, plants were exposed to 5 mM of L-glutamate (Glu), L-cysteine (Cys), or L-serine (Ser) for <2 min and transferred back to 0.53 MS plates to assess regeneration. For ‘‘long’’ treatment, plants were maintained on plates with treatment until regeneration was assessed.
(D) CNQX transient ‘‘window’’ treatments, showing that inhibiting GLRs at the time of injury is critical for enhancing regeneration. Pattern at left shows treatment scheme with white boxes indicating mock and yellow boxes indicating CNQX treatment. Pre, 1 h pretreatment; Cut, cutting plate; 16 h, begins immediately after cutting and ends 16 h later; Reg, begins 16 h after cutting and continues for the duration of the experiment. (Chi-square, n ≥ 40 for each treatment, **p < 0.0008).
(E) The glr quadruple mutant (3) shows enhanced callus formation, with bar graph showing percent of leaves that formed callus for each genotype, scored at 2 weeks post callus initiation. Right, representative images of callus formation in leaves from wild type, triple and quadruple GLR mutants, showing more extensive callus formation in mutants versus. Wild-type leaves that did form callus. In all cases, formation of callus occurred only in some regions of the leaf (partial). The error bars are proportional to the standard error of the pooled percentage computed using binomial distribution (chi-square test; n R 32 for each genotype; **p < 10‒9). Arrows indicate regions of callus formation. Scale bars, 0.5 cm.
(F) Callus formation in leaves treated with the GLR antagonist CNQX is enhanced, showing percent of leaves with callus. Images show roots in both conditions that formed callus. Arrow indicates region of callus formation in mock, while the treated sample is completely transformed to callus, indicating accelerated callus formation. The error bars are proportional to the standard error of the pooled percentage (chi-square test; n > 48 for each treatment; *p < 10‒29) Scale bars, 0.5 cm.
(G) Callus microcolony formation from single cells is enhanced by addition of 1-µM CNQX to the liquid media (chi-square test; n = 8.0 × 104 cells, where frequency of callus formation was estimated from hema-cytometer readings of 3 samples from each of two biological replicates and extrapolated; **p < 10‒30). At right, representative images of microcolony formation. Scale bar, 2 mm.
(H) Root-from-leaf regeneration under CNQX treatment, showing more leaves with roots in CNQX treatment. (Chi-square test; Fisher’s combined probability test, n ≥ 18 for each treatment in each of 6 trials, trial 3, n = 12; **p < 0.0005) Image shows more extensive root system of CNQX-treated leaves. Scale bars, 65 mm.
(I) Root-from-leaf regeneration under CNQX treatment assessed at 13 and 20 days. The difference in de novo root formation between treated and untreated leaves diminished over 20-day incubation (chi-square test; n = 40 for each treatment at each time point; *p < 0.03).
