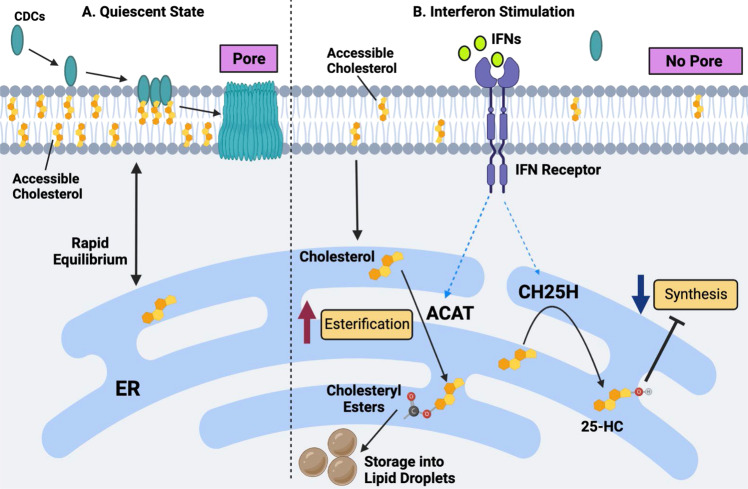
Fig. 4. A working model of interferon-mediated protection of macrophages against CDC-induced cytotoxicity.
A In a quiescent state, CDCs target metabolically active (or accessible) cholesterol in the plasma membrane of macrophages, resulting in pore formation and the subsequent loss of membrane integrity. B IFN stimulation markedly decreases the size of the accessible cholesterol pool, resulting in reduced CDC binding and pore formation on the plasma membrane. Alterations in the accessible cholesterol pool in the plasma membrane are driven by a reduction in cholesterol biosynthesis and heightened cholesterol esterification. The inhibition of cholesterol synthesis and esterification is dependent, in part, on the upregulation of the interferon-stimulated gene, Ch25h, and the production of oxysterol 25HC. 25HC decreases synthesis via the degradation of the HMGCR enzyme and the inhibition of the SREBP2 transcriptional pathway. 25HC also facilitates cholesterol esterification via the ER-residential enzymes ACAT1 and ACAT2.