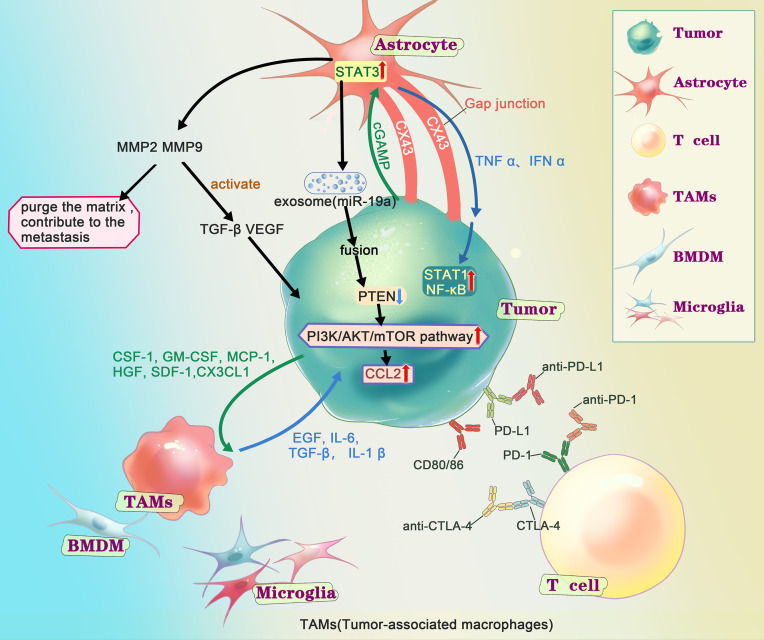
Figure 2.
Interactions of metastatic tumor cells with the brain microenvironment. After the tumor cells get into the CNS (central nervous system), according to the “seed and soil”, series of interactions happened between the tumor cells and the microenvironment in brain. Extensive research has shown that astrocytes play an important role in metastasis through matrix metalloproteinase 2 (MMP2), MMP9, exosome and gap junction, generally supports the growth and invasion. MMP2、MMP9 are associated with tumor growth. On the one hand, it could degrade components of the extracellular matrix and basement membrane, on the other hand, it helps to activate TGF-β and VEGF. Several studies have shown that after fusion of AST-generating exosomes with tumor cells, miRNAs contained in the exosomes cause tumor cells to under-express PTEN and further activate the PI3K/AKT/mTOR pathway, leading to more chemokine ligand 2(CCL2), promoting the growth of tumor. There are gap junctions composed of connexin 43 (Cx43) between tumor cell and astrocyte. Through the gap junction, astrocyte release cytokines such as IFNα and TNFα, activating STAT1 and NF-κB pathways, supporting tumor growth. At the same time, tumor cell could transfer cGAMP to astrocytes, activate the STAT3 pathways. Tumor-associated macrophages and microglia (TAMs), including bone marrow-derived macrophages (BMDMs) and microglia (MG), secret growth factors (EGF, IL6, TGF-β, IL-1β), contribute to the colonization. And the tumor cells release chemokines and cytokines (CSF-1, GM-CSF, MCP-1, HGF, SDF-1, CX3CL) to recruit TAMs towards the tumor cells. There are immune checkpoints expressed on T-cells, such as PD-1 and CTLA-4, which tumor cells could bind and deactivate T-cells, suppressing anti-tumor immunity. Checkpoint immunotherapy use antibodies to inhibit immune checkpoint to prevent the T-cell from deactivation and control the tumor.