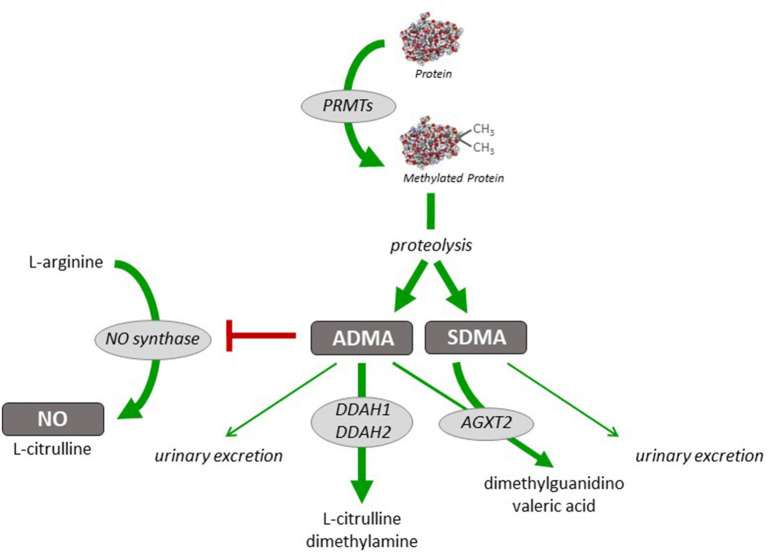
Figure 1.
Schematic representation of pathways of dimethylarginine biosynthesis and metabolism. Dimethylarginines are formed during (di-)methylation of protein-bound L-arginine residues by a family of protein arginine N-methyltransferases (PRMTs). Free ADMA and SDMA are released during physiological hydrolytic protein turnover. Asymmetric dimethylarginine (ADMA) inhibits nitric oxide (NO) synthesis from L-arginine, whilst symmetric dimethylarginine (SDMA) does not directly interfere with NO synthase activity. ADMA is metabolically degraded to L-citrulline and dimethylamine by either of two isoforms of dimethylarginine dimethylaminohydrolase (DDAH). Both ADMA and SDMA can be cleaved by alanine glyoxylate aminotransferase-2 (AGXT2); this enzyme is the major pathway of SDMA clearance. Minor amounts of both ADMA and SDMA can also be excreted into the urine.