Abstract
The long-lasting co-evolution of ticks with pathogens results in mutual adaptation. Blood-feeding is one of the critical physiological behaviors that have been associated with the tick microbiome; however, most knowledge was gained through the study of laboratory-reared ticks. Here we detached Ixodes persulcatus ticks at different stages of blood-feeding from human patients and performed high-throughput transcriptomic analysis on them to identify their virome and genes differentially expressed between flat and fully fed ticks. We also traced bloodmeal sources of those ticks and identified bats and three other potential mammalian hosts, highlighting the public health significance. We found Jingmen tick virus and 13 putative new viruses belonging to 11 viral families, three of which even exhibited high genetic divergence from viruses previously reported in the same tick species from the same geographic region. Furthermore, differential expression analysis suggested a downregulation of antioxidant genes in the fully fed I. persulcatus ticks, which might be related to bloodmeal-related redox homeostasis. Our work highlights the significance of active surveillance of tick viromes and suggests a role of reactive oxygen species (ROS) in modulating changes in the microbiome during blood-feeding.
Keywords: ticks, bloodmeal, virome, reactive oxygen species, patients, Jingmen tick virus
Introduction
Zoonotic pathogens, which reside in animal reservoir species and may spill over into the human population, are emerging at an unprecedented rate (Jones et al., 2008; Shi et al., 2016). Vectors exacerbate and complicate zoonotic disease transmission. Ticks are obligate blood-feeding vectors that parasitize a wide range of animals, including reptiles, birds, and mammals. As a result of their feeding on blood, these arthropods are versatile vectors transmitting a plethora of pathogenic agents, including viruses, bacteria, helminths, and protozoa (de la Fuente et al., 2008; Jia et al., 2020). Blood-feeding is a key physiological process providing essential nutrients for ticks, mainly dietary hemoglobin which is acquired as an exogenous source of heme and thereby sequestered by vitellins in the developing oocytes (Perner et al., 2016). The blood-feeding strategy of ticks provides an ideal system for pathogen transmission, benefiting from their multiple bloodmeals on different hosts to accomplish their complete life cycle and their relatively long-lasting attachment to hosts for several days or weeks (Nava et al., 2009).
Screening of a fed tick detached from a patient is important to evaluate the risk to human health; however, most questions related to this natural, field-collected feeding tick are unsolved. Firstly, identification of the host species on which the tick fed during its previous parasitic stage is imperative to predict the risk of pathogen transmission; however, there are only limited reports of tracing the host species of tick blood meals (Allan et al., 2010; Önder et al., 2013). Secondly, the vector-borne virome has been increasingly highlighted in identifying new pathogens; however, the virome is only available for about 10 tick species (Tokarz et al., 2014, 2018; Pettersson et al., 2017; Harvey et al., 2019; Meng et al., 2019; Vandegrift and Kapoor, 2019) among over 800 tick species worldwide (Jongejan and Uilenberg, 2004). Thirdly, studies have demonstrated that the host bloodmeal can significantly influence the tick microbiome in various ways, which may fundamentally impact the transmission of zoonotic pathogens in these natural systems (Hawlena et al., 2013; Zhang et al., 2014; Rynkiewicz et al., 2015; Swei and Kwan, 2017; Charrier et al., 2018; Zolnik et al., 2018). However, most blood-feeding ticks in previous studies were reared under laboratory conditions, whereas field-collected ticks were rarely analyzed.
To fill the knowledge gaps mentioned above, we detached Ixodes persulcatus at different blood-feeding stages from patients and carried out a transcriptomic analysis. The hard tick I. persulcatus is widely distributed in Northern China and is most commonly responsible for tick-borne diseases in humans in this region (Cao et al., 2000, 2003). Ixodes persulcatus transmits a various disease-causing bacteria, including genera Borrelia, Rickettsia, Anaplasma, Francisella, Coxiella, and Ehrlichia. We aimed to identify the possible animal hosts on which the ticks had fed during the previous parasitic stage, elucidate the virome of detached ticks using transcriptomic approaches, and explore blood meal-related tick responses. Our study may help to identify new biotic drivers indicating new strategies to control ticks and the pathogens they transmit.
Materials and Methods
Tick Collection
Ticks feeding on patients who sought treatment at Mudanjiang Forestry Central Hospital in Heilongjiang Province, China, were collected between May and August 2016. The study was approved by the ethics committee of the hospital (Mudanjiang Forest Central Hospital 2011-03) in accordance with the medical research regulations of China. The species and developmental stage were identified by an entomologist (Yi Sun). Adult I. persulcatus ticks were included in the study, and the species was later confirmed by analyzing sequences of the mitochondrial cytochrome c oxidase subunit I gene. We further divided the feeding ticks into three groups: flat (ticks that had imbibed small amounts of blood and had not started to enlarge), partially fed (ticks that had begun to enlarge but were not fully engorged), and fully fed (ticks that had fully engorged and were almost ready to detach). All samples were captured alive and thoroughly surface-sterilized (two successive washes in 70% ethanol for 30 s each) before storage at −80°C for DNA/RNA extraction.
Sample Preparation and Sequencing
Extraction of total DNA and RNA from ticks was performed using the AllPrep DNA/RNA Mini Kit (Qiagen, Valencia, CA, United States) with modifications. Briefly, ticks were homogenized in RLT solution under liquid nitrogen. The homogenate was then incubated at 55°C for 10 min with proteinase K (Qiagen) and centrifuged for 30 s at 15,000 × g. The homogenized lysate was transferred to an AllPrep DNA spin column and centrifuged for 30 s at 8,000 × g. The AllPrep DNA spin column was used for later DNA purification, and the flow-through was used for RNA purification as per the manufacturer’s instructions. Ten ticks were pooled together to extract RNA/DNA, and each group comprised three pools.
The extracted RNA was used for transcriptome sequencing (RNA-seq) after RNA quantification and quality checking. The rRNA was removed using RiBo-Zero Gold rRNA removal reagents (human/mouse/rat) (Illumina). Then, the sequencing library was prepared following the Illumina standard protocol. Paired-end (2 × 150 bp) sequencing of the RNA library was performed on an Illumina HiSeq 4000 platform at Novogene Tech (Beijing, China).
Discovery and Assembly of Viral Genomes
RNA sequencing reads were de novo assembled using the Trinity program (Grabherr et al., 2011). Assembled contigs were subject to BLASTx against all non-redundant (nr) database downloaded from GenBank, and the threshold E value was set to 1e-5. Putative viral contigs were further merged by high-identity overlaps using the SeqMan program of Lasergene package v7.1 (DNAstar, Madison, WI, United States). Original reads were aligned to the contigs again using Bowtie2 (Langmead and Salzberg, 2012) to complete the remaining gaps, and the assembly was verified in the Integrated Genomics Viewer (Thorvaldsdóttir et al., 2013).
Phylogenetic Analyses
The highly conserved RNA-dependent RNA polymerase (RdRp) gene was used to construct family-level phylogenies comprising the viruses that were highly divergent. Predicted viral proteins of the RdRp genes in this study were aligned with reference proteins of the same viral families using the E-INS-i algorithm in MAFFT version 7 (Katoh and Standley, 2013). Ambiguously aligned regions were removed using TrimAl (Capella-Gutiérrez et al., 2009). The WAG + Γ model was identified as the best-fit amino acid substitution model using Prot-Test 3.4 (Darriba et al., 2011), which was used for maximum likelihood (ML) phylogeny reconstruction with bootstrap tests (1,000 replicates) in PhyML version 3.0 (Guindon and Gascuel, 2003). The ML trees were visualized with FigTree v1.4.2.
Differential Expression Analysis
A single de novo assembly across all samples was generated using Trinity (Grabherr et al., 2011), then reads from each sample were separately aligned back to the single Trinity assembly for downstream analyses of the differential expression of transcripts. Transcript abundance containing RNA-Seq fragment counts for each transcript (or gene) across each sample was estimated using the alignment-based abundance estimation method RSEM (Li et al., 2009). The trimmed mean of M-values normalization method (TMM) were subsequently used to normalize the transcript abundance. Differentially expressed (DE) transcripts or genes were identified by Fisher’s oexact test running “edge” R packages (Robinson et al., 2010; McCarthy et al., 2012), and then those transcripts or genes that had p-values of at most 5e-2 and were at least 2^1.5-fold differentially expressed were extracted to generate a sample correlation matrix heatmap and a DE gene vs. samples heatmap using Trinity downstream analysis script. The DE genes shown in the heatmap were grouped into gene clusters with similar expression patterns by cutting the hierarchically clustered gene tree at 60% from the tip. The functional annotations of the DE transcripts or genes were performed using Trinotate (Bryant et al., 2017) based on Pfam database. We classified these annotated transcripts or genes based on the COG database using eggnog mapper (Huerta-Cepas et al., 2017).
Results
Identification of Bloodmeal Source in Adult Ixodes persulcatus
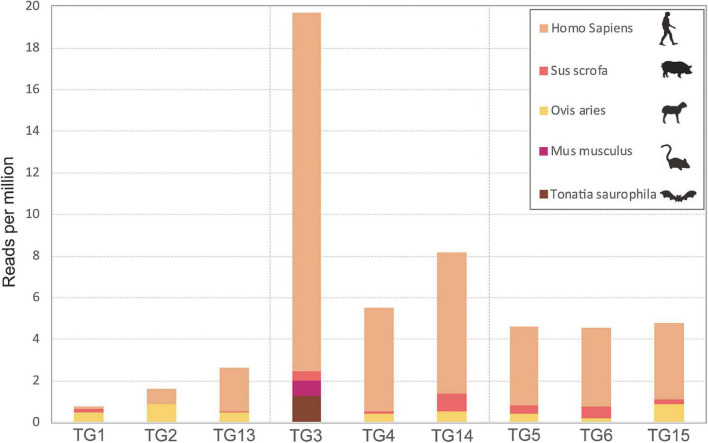
A total of 90 feeding adult I. persulcatus ticks were collected from patients. We sequenced nine pools of ticks (10 ticks/pool) by transcriptomic survey. Pools TG1, TG2, and TG13 comprised flat ticks; pools TG3, TG4, and TG14 comprised partially fed ticks; and pools TG5, TG6, and TG15 comprised fully fed ticks. We analyzed cox1 genes from RNA-seq data to identify the source of previous bloodmeals of I. persulcatus and found five mammal host sources, namely, Tonatia saurophila, Mus musculus, Ovis aries, Sus scrofa, and Homo sapiens (Figure 1). The host cox1 gene sequences assembled in the study had over 98.5% identity to the respective reference sequences (Supplementary Table 1). The most abundant cox1 sequence was from Homo sapiens, accounting for 1,570 reads across all samples. Intriguingly, one bat species, Tonatia saurophila, was detected with a read count of 48 in one of the partially fed tick pools (Figure 1 and Supplementary Table 1).
FIGURE 1.
Expression levels of the cytochrome c oxidase subunit I gene of various host species revealed by RNAseq analysis of pools of Ixodes persulcatus ticks removed from human patients at Mudanjiang Forestry Central Hospital in Heilongjiang Province, China. Ticks were divided into three groups each comprising three pools of ten ticks: flat (pools TG1, TG2, and TG13), partially fed (pools TG3, TG4, and TG14), and fully fed (pools TG5, TG6, and TG15).
Ixodes persulcatus Tick Virome
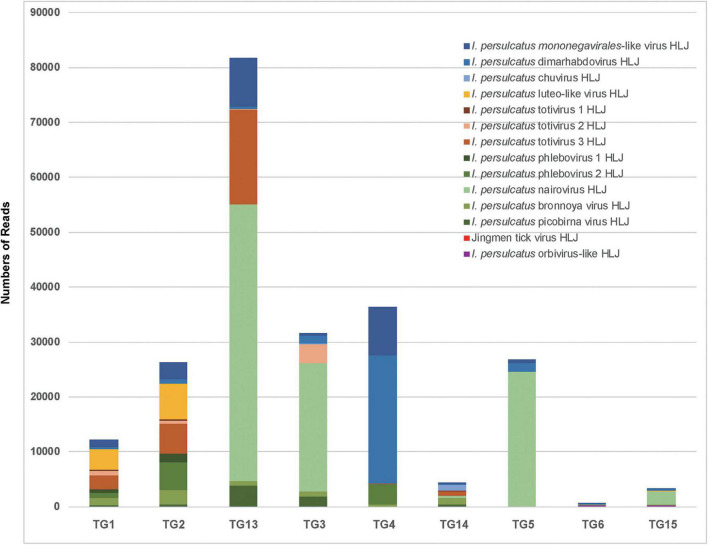
We performed nine RNA-seq runs on each of the nine pools of I. persulcatus ticks, generating 72 GB of total sequence data. Among the nine resultant RNA-seq libraries, library TG13 (flat ticks) had the most abundant viral reads, followed by library TG4 (partially fed ticks), while libraries TG6 and TG15 (fully fed ticks) had the fewest viral reads (Figure 2). Through a BLASTx search with the assembled genome sequences, we discovered 14 known or novel viruses belonging to 11 viral families (Table 1). All of which had fully or almost fully intact genomes with undisrupted reading frames. These included, with proposed names of new viruses in brackets, one Mononegavirales-like virus (I. persulcatus mononegavirales-like virus HLJ), one novel chuvirus (I. persulcatus chuvirus HLJ), one novel rhabdovirus (I. persulcatus dimarhabdovirus HLJ), three distant relatives of Totiviridae (I. persulcatus totivirus 1 HLJ, I. persulcatus totivirus 2 HLJ and I. persulcatus totivirus 3 HLJ), one novel Partitiviridae (I. persulcatus picobirna virus HLJ), one novel orbivirus (I. persulcatus orbivirus-like HLJ), one Luteoviridae-like virus (I. persulcatus luteo-like virus HLJ), four distant relatives of Bunyavirales (I. persulcatus phlebovirus 1 HLJ, I. persulcatus phlebovirus 2 HLJ, I. persulcatus nairovirus HLJ, I. persulcatus bronnoya virus HLJ), and Jingmen tick virus (JMTV). Reads from I. persulcatus nairovirus HLJ were most abundant, followed by reads from I. persulcatus dimarhabdovirus HLJ (Figure 2). These viruses shared 36–99% amino acid identities compared with previously reported viruses, and the query coverage varied from around 30 to 90% (Table 1).
FIGURE 2.
Detection by RNAseq of known and novel viruses in pools of I. persulcatus ticks removed from human patients at Mudanjiang Forestry Central Hospital in Heilongjiang Province, China. Levels of abundance of Jingmen tick virus and 13 newly discovered viruses in flat (pools TG1, TG2, and TG13), partially fed (pools TG3, TG4, and TG14), and fully fed (pools TG5, TG6, and TG15) ticks.
TABLE 1.
Summary of the viruses discovered by RNAseq in Ixodes persulcatus ticks removed from human patients at Mudanjiang Forestry Central Hospital in Heilongjiang Province, China.
| Proposed name | Length | Closest Blastx hit | Identity % | Query Coverage % | Abundance (reads count) |
| Ixodes persulcatus mononegavirales-like virus HLJ | 10,865 | Deer tick mononegavirales-like virus RNA-dependent RNA polymerase gene | 97.64 | 36 | 23,431 |
| Ixodes persulcatus phlebovirus 1 HLJ | 6,696 | Blacklegged tick phlebovirus 3 L gene | 74.66 | 97 | 2,156 |
| Ixodes persulcatus phlebovirus 2 HLJ | 6,715 | Norway phlebovirus 1 L gene | 87.28 | 98 | 9,687 |
| Ixodes persulcatus picobirna virus | 1,639 | Norway partiti-like virus 1 RNA-dependent RNA-polymerase | 93.82 | 91 | 6,825 |
| Ixodes persulcatus totivirus 3 HLJ | 5,615 | Hubei toti-like virus 24 hypothetical protein 2 | 36.97 | 42 | 26,215 |
| Ixodes persulcatus bronnoya virus HLJ | 9,320 | Bronnoya virus RNA-dependent RNA-polymerase |
38.85 | 88 | 7,756 |
| Ixodes persulcatus totivirus 1 HLJ | 2,682 | Lonestar tick totivirus polymerase | 39.8 | 98 | 672 |
| Ixodes persulcatus luteo-like virus HLJ | 2,646 | Norway luteo-like virus 2 RNA-dependent RNA-polymerase | 93.6 | 89 | 10,215 |
| Ixodes persulcatus dimarhabdovirus HLJ | 10,365 | Norway mononegavirus 1 RNA-dependent RNA-polymerase | 49.46 | 61 | 28,533 |
| Ixodes persulcatus totivirus 2 HLJ | 8,914 | Lonestar tick totivirus polymerase | 39.96 | 31 | 4,857 |
| Ixodes persulcatus nairovirus HLJ | 14,779 | Beiji nairovirus RNA-dependent RNA polymerase | 99.43 | 85 | 101,149 |
| Ixodes persulcatus orbivirus-like HLJ | 4,017 | Skunk River virus VP1 gene | 40.26 | 94 | 560 |
| Jingmen tick virus HLJ | 2,313 | Jingmen tick virus NS5-like protein gene | 92.6 | 99 | 84 |
| Ixodes persulcatus chuvirus HLJ | 11,440 | Blacklegged tick chuvirus 2 L gene | 83.07 | 57 | 1,229 |
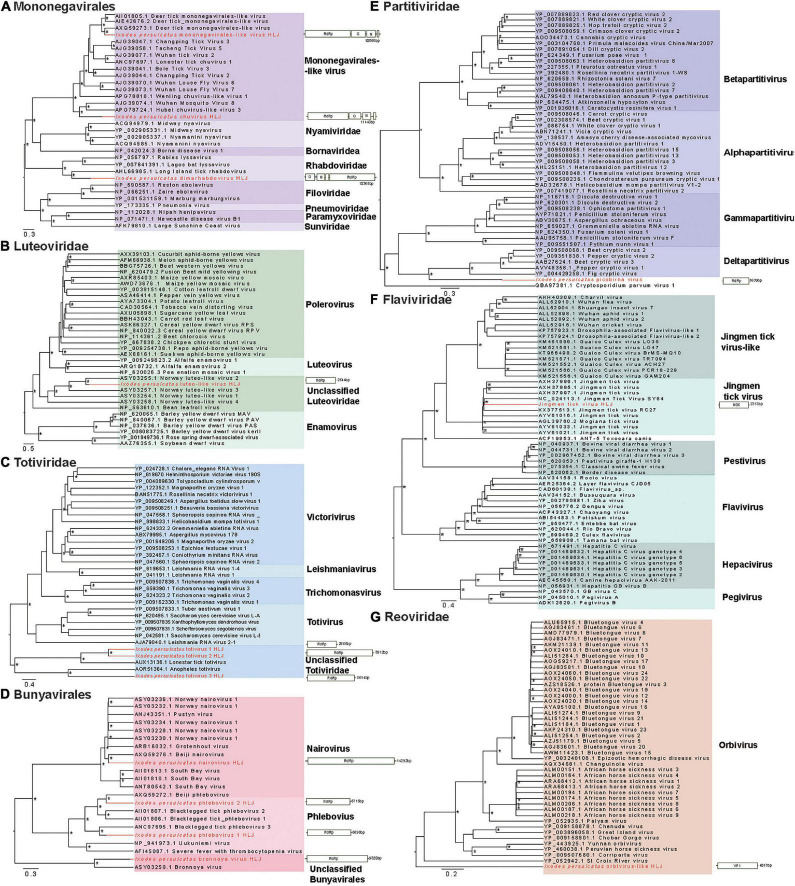
We identified three viruses in the Mononegavirales. Among them, I. persulcatus mononegavirales-like virus HLJ was closely related to deer tick mononegavirales-like virus, which had been previously reported in Heilongjiang Province, China (Meng et al., 2019; Figure 3A). Ixodes persulcatus dimarhabdovirus HLJ clustered with Long Island tick rhabdovirus and formed a monophyletic clade with other rhabdoviruses (Figure 3A). Ixodes persulcatus chuvirus HLJ had 83% amino acid similarity with blacklegged tick chuvirus-2 but only 57% coverage of the L segment sequence. We obtained the nearly complete genomes of these three viruses. The genome of I. persulcatus mononegavirales-like virus HLJ, I. persulcatus dimarhabdovirus HLJ, and I. persulcatus chuvirus HLJ were 10,865, 10,365, and 11,440 nucleotides (nt) long, and all encoded four predicted ORFs.
FIGURE 3.
Phylogenetic trees of the RNA-dependent RNA polymerase (RdRp) gene-based on representative amino acid sequences and schematic genome structures of 14 viruses detected in I. persulcatus ticks removed from human patients at Mudanjiang Forestry Central Hospital in Heilongjiang Province, China. (A) Mononegavirales; (B) Luteoviridae; (C) Totiviridae; (D) Bunyavirales; (E) Partitiviridae; (F) Flaviviridae; and (G) Reoviridae. Support values above 0.7 are indicated by asterisk. The trees were mid-point rooted.
Three distant relatives of Totiviridae (I. persulcatus totivirus 1 HLJ, I. persulcatus totivirus 2 HLJ, I. persulcatus totivirus 3 HLJ) had extremely low amino acid identity (37–40%) with previously described viruses. Their RdRp sequences were highly divergent from the existing clades and fell into the unclassified groups on the phylogenetic tree (Figure 3C). This indicates the distinct phylogenetic position of these newly identified viruses from the currently known viruses.
Three members of the Bunyavirales, I. persulcatus phlebovirus 1 HLJ, I. persulcatus phlebovirus 2 HLJ, and I. persulcatus nairovirus HLJ showed high amino acid identities of 75, 87, and 99% with previously reported blacklegged tick phlebovirus 3, Norway phlebovirus 1, and Beiji nairovirus, respectively, based on the RdRp gene. The fourth member of the Bunyavirales, I. persulcatus bronnoya virus HLJ exhibited 38% amino acid identity with Bronnoya virus (88% sequence coverage of RdRp gene). The phylogenetic tree of Bunyavirales indicated that the two phleboviruses and the nairovirus clustered with previously reported Chinese strains and formed well-supported monophyletic groups closely related to the “classic” phleboviruses and nairoviruses (Figure 3D). It is suggested that they may share a single common ancestor for this gene. The newly discovered Bronnoya virus RdRp sequence clustered with the previously reported Bronnoya virus.
Ixodes persulcatus luteo-like virus HLJ, I. persulcatus picobirna virus HLJ, and JMTV found in this study exhibited 87, 94, and 99% amino acid identities to previously reported viruses with, respectively, 98, 91, and 93% sequence coverage (Figures 3B,E,F).
Ixodes persulcatus orbivirus-like HLJ revealed 40% amino acid similarity across 94% of the VP1 gene sequence of the orbivirus Skunk River virus (MK100569.1), and it showed a phylogenetic position distinct from other orbiviruses in the phylogenetic tree (Figure 3G).
Bloodmeal-Associated Differential Gene Expression Analyses
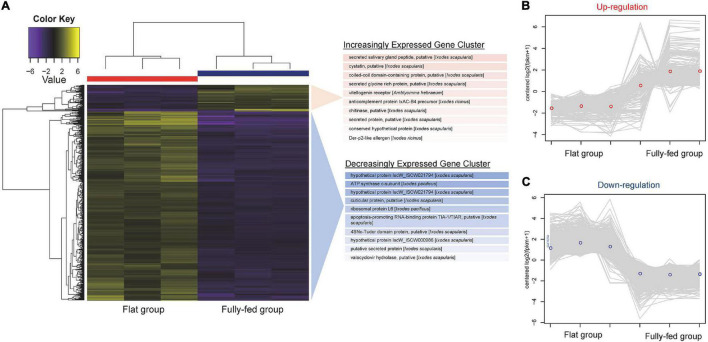
We next detected the differential expression responses of ticks at two different stages of feeding: flat (pools TG1, TG2, and TG13) and fully fed (pools TG5, TG6, and TG15) to investigate the effect of the bloodmeal. We first generated a single Trinity assembly across all pools and then calculated the abundance separately for each pool. The subsequent differential expression analysis showed that a total of 1,302 genes were differently expressed (p < 0.05, logFC > 1.5). Most of the DE genes were negatively correlated (Supplementary Figure 1). As shown in Figure 4A, most of the DE genes (71.4%) were downregulated in fully fed ticks; of these, ATP synthase c-subunit, a putative cuticular protein, ribosomal protein L6, and the putative apoptosis-promoting RNA-binding protein TIA-1/TIAR were the most downregulated (Supplementary Table 2). The gene encoding a putative secreted salivary gland peptide was most upregulated at engorgement, followed by a putative cystatin, putative coiled-coil domain-containing protein, and putative secreted glycine-rich protein (Supplementary Table 2). One antimicrobial peptide, lysozyme, was downregulated in fully fed ticks (Supplementary Table 3).
FIGURE 4.
RNAseq analysis of tick gene transcripts detected in pools of flat and fully fed I. persulcatus ticks removed from human patients at Mudanjiang Forestry Central Hospital in Heilongjiang Province, China. (A) Clustered heatmap of genes differentially expressed (DE) between flat and fully fed ticks. The top 10 most significant genes in the increasing and decreasing expression clusters are shown. The expression patterns of DE genes in each tick pool are shown for (B) upregulated genes and (C) downregulated genes.
All DE genes shown in the heatmap (Figure 4A) were grouped into clusters of genes with similar expression patterns. A total of 251 transcripts were clustered in the upregulation category (Figure 4B), while 930 transcripts were clustered in the downregulation category (Figure 4C). According to the taxonomy classification based on the COG database, 18.7% of DE genes in the upregulated category were related to post-translational modification, protein turnover, and chaperones, and 12.7% of upregulated DE genes displayed functions of translation, ribosomal structure, and biogenesis (Supplementary Figure 2A). Most of the DE genes in the downregulated category were related to functions of nucleotide transport and metabolism (18.2%) and cytoskeleton (18.3%) (Supplementary Figure 2B).
Reactive Oxygen Species
Reactive Oxygen Species (ROS) produced by the mosquito midgut play a role in maintenance of redox homeostasis (Citelli et al., 2007; Hernandez et al., 2019), and previous literatures also suggested that the balance between positive and negative aspects of ROS in ticks should be carefully maintained (Hernandez et al., 2019). To achieve redox homeostasis, the ticks have a complex anti-oxidant system composed of enzymes such as glutathione peroxidase, glutathione-S transferase, and so on (Hernandez et al., 2018, 2020; Kusakisako et al., 2018, 2020). We found that antioxidant genes including glutathione peroxidase, glutathione S-transferase, peroxidase, peroxiredoxin, and protein kinase C were all downregulated in the fully fed ticks compared with the flat ticks (Supplementary Table 3).
Discussion
Blood-feeding is an essential behavior for ticks, and its role is far beyond that of providing nutrition and energy for molting, development, and vitellogenesis. Its requirement for transmission of tick-borne pathogens is another important function. The present study is the first report on the virome of ticks fed on patients who sought medical attention in a sentinel hospital in northeastern China. Using unbiased high-throughput sequencing platforms, we found that five mammalian species had served as hosts for I. persulcatus, including a bat. We also identified Jingmen tick virus and 13 new viruses belonging to 11 viral families. A downregulation of antioxidant genes associated with blood intake was observed in the fed ticks, which might be correlated with maintenance of the redox homeostasis.
It is important to identify the sources of I. persulcatus bloodmeals to aid in assessing the risk to patients of tick-borne disease transmission. Bats are some of the most notorious natural reservoir/hosts for many fatal viruses, such as Ebola virus, SARS virus, and SARS-CoV-2 virus (Calisher et al., 2006; Zhou et al., 2020). We found that some I. persulcatus ticks feeding on humans may have previously imbibed bat blood, highlighting the need for alertness on this tick species for its role in transmitting novel pathogens from bats to humans. Various host cox1 genes were identified in our data, namely mouse, bat, sheep, wild boar, and human, indicating the high risk of interspecies transmission of tick-borne pathogens. Previous studies have developed DNA barcoding (Kent, 2009; Allan et al., 2010; Gariepy et al., 2012) or proteome profiling (Önder et al., 2013) techniques to identify sources of tick blood meals. However, the host identification success was limited due to the heavily degraded remnants of blood. We used the RNA-seq platform to identify the origin of the host bloodmeals of the pooled samples; however, the sensitivity and specificity of this approach should be further evaluated.
We found 14 viruses, belonging to 11 viral families, and most of them showed quite low similarity and query coverage compared with previously found viruses. Meng et al. found five species of viruses, including JMTV, in the same geographic area (Pettersson et al., 2017). We have previously reported that JMTV was pathogenic to humans (Jia et al., 2019), and the known range of this virus has rapidly expanded to cover Africa, South America, the Caribbean, and Europe (Temmam et al., 2019). We will extend our vigilance to other new viruses found in this study for their potential pathogenicity in humans, as these viruses were found in ticks directly detached from human patients. However, whether I. persulcatus is the vector of these viruses remains to be explored. In addition, the possibility of the new viruses being endogenous virus elements (Bell-Sakyi and Attoui, 2016) is very low because all the viruses were found to have fully or almost fully intact genomes, without disrupted reading frames.
Reactive oxygen species level seems to be a typical association with the maintenance of redox homeostasis in hematophagous arthropods, such as ticks (Lara et al., 2005; Citelli et al., 2007) and mosquitoes (Cirimotich et al., 2011; Oliveira et al., 2011). However, ticks might have developed different mechanisms to control ROS level compared with mosquitoes. For example, ticks have a different pathway for heme biosynthesis and require the acquisition of exogenous heme (Perner et al., 2016). Due to their feeding physiology, ticks are exposed to elevated amount of ROS. The ingestion of host blood containing pro-oxidant molecules, such as heme, could catalyze the production of ROS (Graça-Souza et al., 2006). Aside from blood-feeding, many cellular processes and enzyme activity could result in ROS production and eventually increase oxidative stress levels (Hernandez et al., 2019). Recently, carbohydrate metabolic compensation and peroxiredoxin production were reported to be necessary to maintain the redox balance in ticks (Kusakisako et al., 2016; Della Noce et al., 2019). It has been proposed that tick microbiome would be influenced by oxidative stress (Narasimhan and Fikrig, 2015), and different AMP would have different inhibitory consequence on microbial growth (Saito et al., 2009; Zhang et al., 2015). These immune responses in ticks may work together and eventually influence the viral and bacterial composition of a specific host blood meal through a complicated pathway.
On the other hand, the redox situation may indirectly affect pathogen transmission by changing its balance with other microflora in the ticks as suggested in mosquitoes (Cirimotich et al., 2011). We noticed that the gene encoding reeler domain-containing gut protein was significantly upregulated in the fully fed ticks (Supplementary Table 3). A protein of Ixodes scapularis with a Reeler domain (PIXR) was induced upon feeding and upregulated in Borrelia burgdorferi-infected I. scapularis tick guts. PIXR might influence the tick gut microbiome composition by regulating the ability of gram-positive bacteria to form biofilms in the gut when the tick takes a blood meal (Narasimhan et al., 2017). These alterations may influence B. burgdorferi entering the tick gut in multiple ways (Zhang et al., 2015). We identified that the gene encoding a protein similar to PIXR was upregulated in the fully fed I. persulcatus ticks; however, its role in the transmission of Borrelia and other tick-borne pathogens deserves further study.
We noted several limitations as follows: The interpretation of differential gene expression between the flat and fully fed ticks might be over-cautious because we used pooled samples with a relatively limited sample size. Using laboratory-reared ticks from a single colony can decrease the bias caused by individual tick differences; however, this study examined bloodmeal-induced gene regulation and virome in ticks originating from the field and feeding on human patients. We thereby gained valuable information on the risks to patients from tick-borne viruses, at the expense of likely uniformity in response to blood-feeding amongst the studied ticks. We suggest that further research with more field and laboratory-derived samples, and experiments from proteomics and functional genomics perspectives, could be performed to verify our findings.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, PRJNA639641.
Ethics Statement
The studies involving human participants were reviewed and approved by Mudanjiang Forest Central Hospital 2011-03. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
All authors contributed to the manuscript and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all the staff in the tick-borne disease department in Mudanjiang Forestry Central Hospital for their help with sample collections.
Funding
This study was supported by Natural Science Foundation of China (81773492) and the State Key Research Development Program of China (2019YFC1200202). LB-S was funded by the United Kingdom Biotechnology and Biological Sciences Research Council grant BB/P024270/1.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2021.728996/full#supplementary-material
RNAseq analysis of tick gene transcripts detected in pools of flat and fully fed Ixodes persulcatus ticks removed from human patients at Mudanjiang Forestry Central Hospital in Heilongjiang Province, China. Heatmap with clusters showing the Pearson correlation matrix for pairwise sample comparisons of differentially expressed genes in flat (pools TG1, TG2, and TG13), and fully fed (pools TG5, TG6, and TG15) ticks.
RNAseq comparison of tick gene transcripts detected in pools of flat and fully fed I. persulcatus ticks removed from human patients at Mudanjiang Forestry Central Hospital in Heilongjiang Province, China. Taxonomic classification of differentially expressed genes with similar expression patterns based on the Clusters of Orthologous Groups (COG) database (A) COG classification of genes upregulated in fed versus unfed ticks; (B) COG classification of genes downregulated in fed versus unfed ticks.
References
- Allan B. F., Goessling L. S., Storch G. A., Thach R. E. (2010). Blood meal analysis to identify reservoir hosts for Amblyomma americanum ticks. Emerg. Infect. Dis. 16 433–440. 10.3201/eid1603.090911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell-Sakyi L., Attoui H. (2016). Virus Discovery Using Tick Cell Lines. Evol. Bioinform. Online 12 31–34. 10.4137/EBO.S39675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant D. M., Johnson K., DiTommaso T., Tickle T., Couger M. B., Payzin-Dogru D., et al. (2017). A tissue-mapped axolotl de novo transcriptome enables identification of limb regeneration factors. Cell Rep. 18 762–776. 10.1016/j.celrep.2016.12.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calisher C. H., Childs J. E., Field H. E., Holmes K. V., Schountz T. (2006). Bats: important reservoir hosts of emerging viruses. Clin. Microbiol. Rev. 19 531–545. 10.1128/CMR.00017-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W. C., Zhao Q. M., Zhang P. H., Dumler J. S., Zhang X. T., Fang L. Q., et al. (2000). Granulocytic Ehrlichiae in Ixodes persulcatus ticks from an area in China where Lyme disease is endemic. J. Clin. Microbiol. 38 4208–4210. 10.1128/JCM.38.11.4208-4210.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W. C., Zhao Q. M., Zhang P. H., Yang H., Wu X. M., Wen B. H., et al. (2003). Prevalence of Anaplasma phagocytophila and Borrelia burgdorferi in Ixodes persulcatus ticks from northeastern China. Am. J. Trop. Med. Hyg. 68 547–550. 10.4269/ajtmh.2003.68.547 [DOI] [PubMed] [Google Scholar]
- Capella-Gutiérrez S., Silla-Martínez J. M., Gabaldón T. (2009). trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25 1972–1973. 10.1093/bioinformatics/btp348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrier N. P., Couton M., Voordouw M. J., Rais O., Durand-Hermouet A., Hervet C., et al. (2018). Whole body transcriptomes and new insights into the biology of the tick Ixodes ricinus. Parasit. Vectors 11:364. 10.1186/s13071-018-2932-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirimotich C. M., Dong Y., Clayton A. M., Sandiford S. L., Souza-Neto J. A., Mulenga M., et al. (2011). Natural microbe-mediated refractoriness to Plasmodium infection in Anopheles gambiae. Science 332 855–858. 10.1126/science.1201618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citelli M., Lara F. A., da Silva Vaz I., Jr., Oliveira P. L. (2007). Oxidative stress impairs heme detoxification in the midgut of the cattle tick, Rhipicephalus (Boophilus) microplus. Mol. Biochem. Parasitol. 151 81–88. 10.1016/j.molbiopara.2006.10.008 [DOI] [PubMed] [Google Scholar]
- Darriba D., Taboada G. L., Doallo R., Posada D. (2011). ProtTest 3: fast selection of best-fit models of protein evolution. Bioinformatics 27 1164–1165. 10.1093/bioinformatics/btr088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente J., Estrada-Pena A., Venzal J. M., Kocan K. M., Sonenshine D. E. (2008). Overview: ticks as vectors of pathogens that cause disease in humans and animals. Front. Biosci. 13 6938–6946. 10.2741/3200 [DOI] [PubMed] [Google Scholar]
- Della Noce B., Carvalho Uhl M. V., Machado J., Waltero C. F., de Abreu L. A., da Silva R. M., et al. (2019). Carbohydrate Metabolic Compensation Coupled to High tolerance to oxidative stress in ticks. Sci. Rep. 9:4753. 10.1038/s41598-019-41036-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariepy T. D., Lindsay R., Ogden N., Gregory T. R. (2012). Identifying the last supper: utility of the DNA barcode library for bloodmeal identification in ticks. Mol. Ecol. Resour. 12 646–652. 10.1111/j.1755-0998.2012.03140.x [DOI] [PubMed] [Google Scholar]
- Grabherr M. G., Haas B. J., Yassour M., Levin J. Z., Thompson D. A., Amit I., et al. (2011). Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 29 644–652. 10.1038/nbt.1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graça-Souza A. V., Maya-Monteiro C., Paiva-Silva G. O., Braz G. R., Paes M. C., Sorgine M. H., et al. (2006). Adaptations against heme toxicity in blood-feeding arthropods. Insect Biochem. Mol. Biol. 36 322–335. 10.1016/j.ibmb.2006.01.009 [DOI] [PubMed] [Google Scholar]
- Guindon S., Gascuel O. (2003). A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52 696–704. 10.1080/10635150390235520 [DOI] [PubMed] [Google Scholar]
- Harvey E., Rose K., Eden J. S., Lo N., Abeyasuriya T., Shi M., et al. (2019). Extensive diversity of RNA viruses in Australian ticks. J. Virol. 93 e01358–18. 10.1128/JVI.01358-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawlena H., Rynkiewicz E., Toh E., Alfred A., Durden L. A., Hastriter M. W., et al. (2013). The arthropod, but not the vertebrate host or its environment, dictates bacterial community composition of fleas and ticks. ISME J. 7 221–223. 10.1038/ismej.2012.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez E. P., Kusakisako K., Talactac M. R., Galay R. L., Hatta T., Matsuo T., et al. (2018). Characterization and expression analysis of a newly identified glutathione S-transferase of the hard tick Haemaphysalis longicornis during blood-feeding. Parasit. Vectors 11:91. 10.1186/s13071-018-2667-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez E. P., Shimazaki K., Niihara H., Umemiya-Shirafuji R., Fujisaki K., Tanaka T. (2020). Expression analysis of glutathione S-transferases and ferritins during the embryogenesis of the tick Haemaphysalis longicornis. Heliyon 6:e03644. 10.1016/j.heliyon.2020.e03644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez E. P., Talactac M. R., Fujisaki K., Tanaka T. (2019). The case for oxidative stress molecule involvement in the tick-pathogen interactions -an omics approach. Dev. Comp. Immunol. 100:103409. 10.1016/j.dci.2019.103409 [DOI] [PubMed] [Google Scholar]
- Huerta-Cepas J., Forslund K., Coelho L. P., Szklarczyk D., Jensen L. J., von Mering C., et al. (2017). Fast genome-wide functional annotation through orthology assignment by eggNOG-mapper. Mol. Biol. Evol. 34 2115–2122. 10.1093/molbev/msx148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia N., Liu H. B., Ni X. B., Bell-Sakyi L., Zheng Y. C., Song J. L., et al. (2019). Emergence of human infection with Jingmen tick virus in China: a retrospective study. EBioMedicine 43 317–324. 10.1016/j.ebiom.2019.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia N., Wang J., Shi W., Du L., Sun Y., Zhan W., et al. (2020). Large-Scale Comparative Analyses of Tick Genomes Elucidate Their Genetic Diversity and Vector Capacities. Cell 182 1328–1340.e13. 10.1016/j.cell.2020.07.023 [DOI] [PubMed] [Google Scholar]
- Jones K. E., Patel N. G., Levy M. A., Storeygard A., Balk D., Gittleman J. L., et al. (2008). Global trends in emerging infectious diseases. Nature 451 990–993. 10.1038/nature06536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongejan F., Uilenberg G. (2004). The global importance of ticks. Parasitology 129 S3–S14. 10.1017/s0031182004005967 [DOI] [PubMed] [Google Scholar]
- Katoh K., Standley D. M. (2013). MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30 772–780. 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent R. J. (2009). Molecular methods for arthropod bloodmeal identification and applications to ecological and vector-borne disease studies. Mol. Ecol. Resour. 9 4–18. 10.1111/j.1755-0998.2008.02469.x [DOI] [PubMed] [Google Scholar]
- Kusakisako K., Galay R. L., Umemiya-Shirafuji R., Hernandez E. P., Maeda H., Talactac M. R., et al. (2016). 2-Cys peroxiredoxin is required in successful blood-feeding, reproduction, and antioxidant response in the hard tick Haemaphysalis longicornis. Parasit. Vectors 9:457. 10.1186/s13071-016-1748-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusakisako K., Hernandez E. P., Talactac M. R., Yoshii K., Umemiya-Shirafuji R., Fujisaki K., et al. (2018). Peroxiredoxins are important for the regulation of hydrogen peroxide concentrations in ticks and tick cell line. Ticks Tick Borne Dis. 9 872–881. 10.1016/j.ttbdis.2018.03.016 [DOI] [PubMed] [Google Scholar]
- Kusakisako K., Morokuma H., Talactac M. R., Hernandez E. P., Yoshii K., Tanaka T. A. (2020). Peroxiredoxin From the Haemaphysalis longicornis Tick Affects Langat Virus Replication in a Hamster Cell Line. Front. Cell. Infect. Microbiol. 10:7. 10.3389/fcimb.2020.00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B., Salzberg S. L. (2012). Fast gapped-read alignment with Bowtie 2. Nat. Methods 9 357–359. 10.1038/nmeth.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara F. A., Lins U., Bechara G. H., Oliveira P. L. (2005). Tracing heme in a living cell: hemoglobin degradation and heme traffic in digest cells of the cattle tick Boophilus microplus. J. Exp. Biol. 208 3093–3101. 10.1242/jeb.01749 [DOI] [PubMed] [Google Scholar]
- Li B., Ruotti V., Stewart R. M., Thomson J. A., Dewey C. N. (2009). RNA-Seq gene expression estimation with read mapping uncertainty. Bioinformatics 26 493–500. 10.1093/bioinformatics/btp692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy D. J., Chen Y., Smyth G. K. (2012). Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res. 40 4288–4297. 10.1093/nar/gks042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng F., Ding M., Tan Z., Zhao Z., Xu L., Wu J., et al. (2019). Virome analysis of tick-borne viruses in Heilongjiang Province, China. Ticks Tick-Borne Dis. 10 412–420. 10.1016/j.ttbdis.2018.12.002 [DOI] [PubMed] [Google Scholar]
- Narasimhan S., Fikrig E. (2015). Tick microbiome: the force within. Trends Parasitol. 31 315–323. 10.1016/j.pt.2015.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan S., Schuijt T. J., Abraham N. M., Rajeevan N., Coumou J., Graham M., et al. (2017). Modulation of the tick gut milieu by a secreted tick protein favors Borrelia burgdorferi colonization. Nat. Commun. 8:184. 10.1038/s41467-017-00208-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nava S., Guglielmone A. A., Mangold A. J. (2009). An overview of systematics and evolution of ticks. Front. Biosci. 14 2857–2877. 10.2741/3418 [DOI] [PubMed] [Google Scholar]
- Oliveira J. H., Gonçalves R. L., Lara F. A., Dias F. A., Gandara A. C., Menna-Barreto R. F., et al. (2011). Blood meal-derived heme decreases ROS levels in the midgut of Aedes aegypti and allows proliferation of intestinal microbiota. PLoS Pathog. 7:e1001320. 10.1371/journal.ppat.1001320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Önder Ö., Shao W., Kemps B. D., Lam H., Brisson D. (2013). Identifying sources of tick blood meals using unidentified tandem mass spectral libraries. Nat. Commun. 4:1746. 10.1038/ncomms2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perner J., Sobotka R., Sima R., Konvickova J., Sojka D., Oliveira P. L., et al. (2016). Acquisition of exogenous haem is essential for tick reproduction. Elife 5:e12318. 10.7554/eLife.12318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson J. H., Shi M., Bohlin J., Eldholm V., Brynildsrud O. B., Paulsen K. M., et al. (2017). Characterizing the virome of Ixodes ricinus ticks from northern Europe. Sci. Rep. 7:10870. 10.1038/s41598-017-11439-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M. D., McCarthy D. J., Smyth G. K. (2010). edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26 139–140. 10.1093/bioinformatics/btp616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rynkiewicz E. C., Hemmerich C., Rusch D. B., Fuqua C., Clay K. (2015). Concordance of bacterial communities of two tick species and blood of their shared rodent host. Mol. Ecol. 24 2566–2579. 10.1111/mec.13187 [DOI] [PubMed] [Google Scholar]
- Saito Y., Konnai S., Yamada S., Imamura S., Nishikado H., Ito T., et al. (2009). Identification and characterization of antimicrobial peptide, defensin, in the taiga tick, Ixodes persulcatus. Insect Mol. Biol. 18 531–539. 10.1111/j.1365-2583.2009.00897.x [DOI] [PubMed] [Google Scholar]
- Shi M., Lin X. D., Tian J. H., Chen L. J., Chen X., Li C. X. (2016). Redefining the invertebrate RNA virosphere. Nature 540 539–543. 10.1038/nature20167 [DOI] [PubMed] [Google Scholar]
- Swei A., Kwan J. Y. (2017). Tick microbiome and pathogen acquisition altered by host blood meal. ISME J. 11 813–816. 10.1038/ismej.2016.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temmam S., Bigot T., Chrétien D., Gondard M., Pérot P., Pommelet V., et al. (2019). Insights into the Host Range, Genetic Diversity, and Geographical Distribution of Jingmenviruses. mSphere 4 e00645–19. 10.1128/mSphere.00645-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorvaldsdóttir H., Robinson J. T., Mesirov J. P. (2013). Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief. Bioinform. 14 178–192. 10.1093/bib/bbs017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokarz R., Sameroff S., Tagliafierro T., Jain K., Williams S. H., Cucura D. M., et al. (2018). Identification of novel viruses in Amblyomma americanum, Dermacentor variabilis, and Ixodes scapularis ticks. mSphere 3 e00614–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokarz R., Williams S. H., Sameroff S., Sanchez Leon M., Jain K., Lipkin W. I. (2014). Virome analysis of Amblyomma americanum, Dermacentor variabilis, and Ixodes scapularis ticks reveals novel highly divergent vertebrate and invertebrate viruses. J. Virol. 88 11480–11492. 10.1128/JVI.01858-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandegrift K. A.-O., Kapoor A. A.-O. (2019). The Ecology of New Constituents of the Tick Virome and Their Relevance to Public Health. Viruses 11:529. 10.3390/v11060529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Yang S., Gong H., Cao J., Zhou Y., Zhou J. (2015). Functional analysis of a novel cysteine-rich antimicrobial peptide from the salivary glands of the tick Rhipicephalus haemaphysaloides. Parasitol. Res. 114 3855–3863. 10.1007/s00436-015-4615-8 [DOI] [PubMed] [Google Scholar]
- Zhang X. C., Yang Z. N., Lu B., Ma X. F., Zhang C. X., Xu H. J. (2014). The composition and transmission of microbiome in hard tick, Ixodes persulcatus, during blood meal. Ticks Tick-Borne Dis. 5 864–870. 10.1016/j.ttbdis.2014.07.009 [DOI] [PubMed] [Google Scholar]
- Zhou P., Yang X. L., Wang X. G., Hu B., Zhang L., Zhang W., et al. (2020). A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579 270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolnik C. P., Falco R. C., Daniels T. J., Kolokotronis S. O. (2018). Transient influence of blood meal and natural environment on blacklegged tick bacterial communities. Ticks Tick-Borne Dis. 9 563–572. 10.1016/j.ttbdis.2018.01.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
RNAseq analysis of tick gene transcripts detected in pools of flat and fully fed Ixodes persulcatus ticks removed from human patients at Mudanjiang Forestry Central Hospital in Heilongjiang Province, China. Heatmap with clusters showing the Pearson correlation matrix for pairwise sample comparisons of differentially expressed genes in flat (pools TG1, TG2, and TG13), and fully fed (pools TG5, TG6, and TG15) ticks.
RNAseq comparison of tick gene transcripts detected in pools of flat and fully fed I. persulcatus ticks removed from human patients at Mudanjiang Forestry Central Hospital in Heilongjiang Province, China. Taxonomic classification of differentially expressed genes with similar expression patterns based on the Clusters of Orthologous Groups (COG) database (A) COG classification of genes upregulated in fed versus unfed ticks; (B) COG classification of genes downregulated in fed versus unfed ticks.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, PRJNA639641.